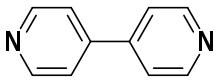
4,4'-Bipyridine
4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is a bipyridine which is mainly used as a precursor to N,N′-dimethyl-4,4′-bipyridinium [(C5H4NCH3)2]2+, known as paraquat. This species is electroactive, and its toxicity arises from the ability of this dication to interrupt biological electron transfer. Because of its structure, 4,4′-bipyridine can bridge between metal centres to give coordination polymers.[1] 4,4′-Bipyridine can also mediate electronic effects between two paramagnetic metal centers.[2]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4′-Bipyridine | |
| Identifiers | |
3D model (JSmol) |
|
| 113176 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.216 |
| EC Number |
|
| 3759 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8N2 | |
| Molar mass | 156.188 g·mol−1 |
| Melting point | 114 °C (237 °F; 387 K) |
| Boiling point | 305 °C (581 °F; 578 K) |
| Structure | |
| 0 D | |
| Related compounds | |
Related compounds |
2,2′-Bipyridine Pyridine 4-Pyridylnicotinamide Terpyridine Biphenyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
4,4′-Bipyridine was first obtained in 1868 by the Scottish chemist Thomas Anderson (1819–1874) via heating pyridine with sodium metal.[3] However, Anderson's empirical formula for 4,4′-bipyridine was incorrect.[4] The correct empirical formula, and the correct molecular structure, for 4,4′-bipyridine was provided in 1882 by the Austrian chemist Hugo Weidel and his student M. Russo.[5]
Uses
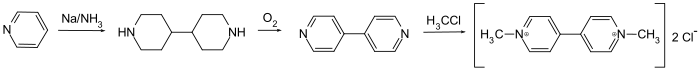
4,4'-Bipyridine is an intermediate in the production of paraquat, a widely-used herbicide. In this process, pyridine is oxidized to 4,4'-bipyridine in a coupling reaction, followed by dimethylation to form paraquat.[6]
References
- Biradha, K.; Sarkar, M.; Rajput, L. (2006). "Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms". Chemical Communications (40): 4169–79. doi:10.1039/B606184B. PMID 17031423.
- Zeng, M.-H.; Zhang, W.-X.; Sun, X.-Z.; Chen, X.-M. (2005). "Spin Canting and Metamagnetism in a 3D Homometallic Molecular Material Constructed by Interpenetration of Two Kinds of Cobalt(II)-Coordination-Polymer Sheets". Angewandte Chemie International Edition. 44 (20): 3079. doi:10.1002/anie.200462463. PMID 15770632.
- See:
- Anderson, Thomas (1868). "On the products of the destructive distillation of animal substances. Part V." Transactions of the Royal Society of Edinburgh. 25: 205–216. Anderson called 4,4′-bipyridine "Dipyridine".
- German translation: Anderson, Th. (1870). "Ueber die Producte der trockenen Destillation thierischer Materien. Fünfter Theil" [On the products of the dry distillation of animal materials. Fifth part.]. Annalen der Chemie und Pharmacie (in German). 154: 270–286.
- See also: Fehling, Hermann Christian von, ed. (1890). Neues Handwörterbuch der Chemie [New Concise Dictionary of Chemistry] (in German). vol. 5. Braunschweig, Germany: Friedrich Vieweg und Sohn. p. 974. See γ-Dipyridyl.
- Anderson gave the empirical formula for 4,4′-bipyridine as C10H10N2. See:
- (Anderson, 1868), p. 209.
- (Fehling, 1890), p. 974 (γ-Dipyridyl).
- Weidel, H.; Russo, M. (1882). "Studien über das Pyridin" [Studies of pyridine]. Monatshefte für Chemie (in German). 3: 850–885. The empirical formula for 4,4′-bipyridine (γ-Dipyridyl) appears on p. 856 ; the molecular structure of 4,4′-bipyridine (γ-Dipyridyl) appears on p. 867.
- "Paraquat and Diquat". IPCS INCHEM.