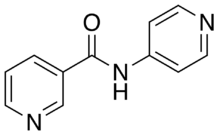
4-Pyridylnicotinamide
4-Pyridylnicotinamide (4-PNA), also known as N-(pyridin-4-yl)nicotinamide, is a kinked dipodal dipyridine which was originally developed for use in chemotherapy.[1] As in its isomer 3-pyridylnicotinamide, the nitrogen atoms on its pyridine rings can donate their electron lone pairs to metal cations, allowing it to bridge metal centers and act as a bidentate ligand in coordination polymers.[2][3][4][5] It is synthesized through the reaction of nicotinoyl chloride and 4-aminopyridine.[1]
 | |
| Names | |
|---|---|
| IUPAC name
N-Pyridin-4-ylpyridine-3-carboxamide | |
| Other names
4-PNA; N-4-Pyridinyl-3-pyridinecarboxamide,; N-(Pyridin-4-yl)nicotinamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C11H9N3O | |
| Molar mass | 199.213 g·mol−1 |
| Density | 1.287 g/cm3 |
| Boiling point | 286.08 °C (546.94 °F; 559.23 K) |
| Structure | |
| 0 D | |
| Related compounds | |
Related compounds |
4,4'-bipyridine Pyridine Nicotinamide 4-Aminopyridine 3-Pyridylnicotinamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Gardner, T. S.; Wenis, E.; Lee, J. (1954). "The Synthesis of Compounds for the Chemotherapy of Tuberculosis. Iv. The Amide Function". The Journal of Organic Chemistry. 19 (5): 753. doi:10.1021/jo01370a009.
- Kraft, P. E.; Laduca, R. L. (2012). "Catena-Poly\\tetra-μ-benzoato-κ8O:O′-dicopper(II)]-μ-\N-(pyridin-4-yl)nicotinamide]-κ2N:N′-\dibenzoato-κ2O-copper(II)]-μ-\N-(pyridin-4-yl)nicotinamide]-κ2N:N′]". Acta Crystallographica Section E. 68 (8): m1049–m1050. doi:10.1107/S1600536812030437. PMC 3414118. PMID 22904725.
- Krishna Kumar, D. (2009). "Exploring the effect of chain length of bridging ligands in coordination complexes and polymers derived from mixed ligand systems of pyridylnicotinamides and dicarboxylates". Inorganica Chimica Acta. 362 (6): 1767–2013. doi:10.1016/j.ica.2008.08.033.
- Kumar, D. K.; Das, A.; Dastidar, P. (2006). "One-Dimensional Chains, Two-Dimensional Corrugated Sheets Having a Cross-Linked Helix in Metal−Organic Frameworks: Exploring Hydrogen-Bond Capable Backbones and Ligating Topologies in Mixed Ligand Systems". Crystal Growth & Design. 6 (8): 1903. doi:10.1021/cg0600344.
- Kumar, D. K.; Das, A.; Dastidar, P. (2006). "Exploring hydrogen-bond capable backbone and ligating topologies: Co(II) coordination polymers derived from mixed ligand systems". Journal of Molecular Structure. 796 (1–3): 139–145. Bibcode:2006JMoSt.796..139K. doi:10.1016/j.molstruc.2006.02.033.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.