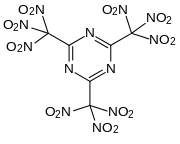
2,4,6-Tris(trinitromethyl)-1,3,5-triazine
2,4,6-Tris(trinitromethyl)-1,3,5-triazine is a chemical compound that is a derivative of triazine first prepared in 1995.[1] It is synthesized by destructive nitration of 2,4,6-tricarboxyl-1,3,5-triazine. It is noteworthy for having more nitro groups than it does carbon atoms, so could be used as an oxygen source, or added to oxygen-poor explosives to increase their power.
 | |
-1%2C3%2C5-triazine-3D-balls.png) | |
| Names | |
|---|---|
| IUPAC name
2,4,6-Tris(trinitromethyl)-1,3,5-triazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C6N12O18 | |
| Molar mass | 528.132 g·mol−1 |
| Density | 1.91 g/cm3 |
| Melting point | 91 to 92 °C (196 to 198 °F; 364 to 365 K) |
| Related compounds | |
Related compounds |
4,4’-Dinitro-3,3’-diazenofuroxan Cyanuric triazide Hexanitrohexaazaisowurtzitane Octanitrocubane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Derivatives have been prepared by nucleophilic displacement of the nitro groups with azide and hydrazine.[2]
References
- Shastin, Alexey V.; Godovikova, Tamara I.; Golova, Svetlana P.; Kuz'Min, Vladimir S.; Khmel'Nitskii, Lenor I.; Korsunskii, Boris L. (1995). "Synthesis of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine". Mendeleev Communications. 5: 17. doi:10.1070/MC1995v005n01ABEH000440.
- Shastin, A. V. (2003). "Nucleophilic Substitution Reactions of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine. 3. Reaction of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine with Azides and Hydrazine". Chemistry of Heterocyclic Compounds. 39 (3): 354. doi:10.1023/A:1023970928207.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.