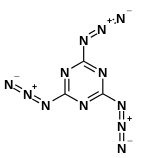
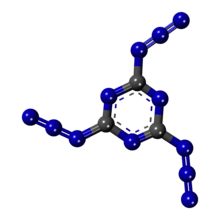
Cyanuric triazide
Cyanuric triazide (C3N12 or (NCN3)3) is described as an environmentally friendly, low toxicity, and organic primary explosive with a detonation velocity of about 7,300 m·s−1, and ignition temperature at 205 °C. Primary research on this compound focuses on its use as a high energy density compound.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,4,6-Triazido-1,3,5-triazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3N12 | |
| Molar mass | 204.117 g·mol−1 |
| Appearance | white crystal |
| Density | 1.73 g·cm−3[1] |
| Melting point | 94 °C (201 °F; 367 K) |
| Boiling point | 150 °C (302 °F; 423 K) Decomposition or explosion |
| Hazards | |
| Main hazards | Flammable and irritant |
| GHS pictograms |  |
| Flash point | [2] |
| 205 °C (401 °F; 478 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
Cyanuric triazide is planar and has three-fold axis symmetry consisting of alternating carbon and nitrogen atoms, whose centers alternate 1.38 Å and 1.31 Å apart. The distance between the center of the ring and carbon atoms of each of the nitrogens is 1.30 Å and 1.39 Å. This fixing of the position of the bonds in the cyanuric ring is mainly due to the unsymmetrical positions of the azide chains. Azide groups are linked to the carbon atoms on the cyanuric ring by single bonds with an interatomic distance of 1.38 Å, similar to the cyanuric ring itself.
Occurrence
This compound is purely synthetic and therefore does not exist in nature.
Synthesis
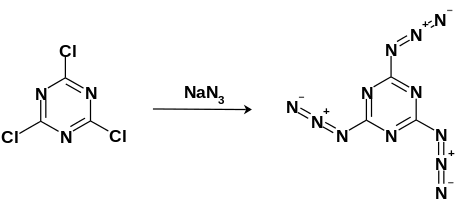
Cyanuric triazide can be synthesized via nucleophilic aromatic substitution using cyanuric trichloride with an excess of sodium azide refluxed in acetone solution. The white crystals can then be purified via crystallization in -20 °C toluene.[3]
Decomposition reactions
This white polycrystalline solid was found to be stable under standard conditions but is extremely shock sensitive causing it to violently decompose when ground with a mortar. The thermodynamic properties of cyanuric triazide were studied using bomb calorimetry with a combustion enthalpy (H) of 2234 kJ·mol−1 under oxidizing conditions and 740 kJ·mol−1 otherwise. The former value is comparable to the military explosive RDX, (C3N3)(NO3)3H6, but is not put into use due to its less than favorable stability. Melting point examination showed a sharp melting range to clear liquid at 94-95 °C, gas evolution at 155 °C, orange to brown solution discoloration at 170 °C, orange-brown solidification at 200 °C and rapid decomposition at 240 °C. The rapid decomposition at 240 °C results from the formation of elemental carbon as graphite and the formation of nitrogen gas.[3]
References
- Haynes, W. M.; Lide, D. R. (2012). CRC Handbook of Chemistry and Physics 93rd Ed. CRC Press/Taylor and Francis. ISBN 1439880492.
- Mikhail A. Ilyushin, Igor V. Tselinsky And Irina V. Shugalei (2012). "Environmentally Friendly Energetic Materials for Initiation Devices" (PDF). Central European Journal of Energetic Materials. 9 (4): 293–327.
- Gillan, Edward G. (2000). "Synthesis of Nitrogen-Rich Carbon Nitride Networks from an Energetic Molecular Azide Precursor". Chemistry of Materials. 12 (12): 3906. doi:10.1021/cm000570y.