Ruxolitinib
Ruxolitinib (trade names Jakafi and Jakavi) is a drug for the treatment of intermediate or high-risk myelofibrosis, a type of myeloproliferative disorder that affects the bone marrow,[2][3] and for polycythemia vera (PCV) when there has been an inadequate response to or intolerance of hydroxyurea.[4][5] Ruxolitinib has also been shown to improve cases of chronic graft versus host disease in patients following a bone marrow transplant. It was developed and is currently sold by Incyte Corp in the US under the brand name Jakafi, and by Novartis elsewhere in the world, under the brand name Jakavi.
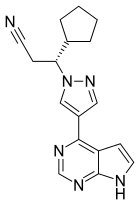 | |
| Clinical data | |
|---|---|
| Pronunciation | Jakafi /ˈdʒækəfaɪ/ JAK-ə-fye |
| Trade names | Jakafi, Jakavi |
| Other names | INCB018424, INC424 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612006 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95%[1] |
| Protein binding | 97%[1] |
| Metabolism | Hepatic (mainly CYP3A4-mediated)[1] |
| Elimination half-life | 2.8-3 hours[1] |
| Excretion | Urine (74%), faeces (22%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H18N6 |
| Molar mass | 306.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of action
Ruxolitinib is a janus kinase inhibitor (JAK inhibitor) with selectivity for subtypes JAK1 and JAK2.[6][7] Ruxolitinib inhibits dysregulated JAK signaling associated with myelofibrosis. JAK1 and JAK2 recruit signal transducers and activators of transcription (STATs) to cytokine receptors leading to modulation of gene expression.
Side effects
Side effects include pancytopenia, thrombocytopenia (low blood platelet count), anemia (low red blood cell count) and neutropenia; risk of infection; symptom exacerbation if the medication is interrupted or discontinued; and non-melanoma skin cancer.[4][8]
Immunologic side effects have included herpes zoster (shingles) and case reports of opportunistic infections.[9] Metabolic side effects have included weight gain. Laboratory abnormalities have included alanine transaminase (ALT) abnormalities, aspartate transaminase (AST) abnormalities, and mildly elevated cholesterol levels.[4]
Approval
The phase III Controlled Myelofibrosis Study with Oral JAK Inhibitor-I (COMFORT-I) and COMFORT-II trials showed significant benefits by reducing spleen size and relieving debilitating symptoms.[10][11][12][13]
In November 2011, ruxolitinib was approved by the U.S. Food and Drug Administration (FDA) for the treatment of intermediate or high-risk myelofibrosis based on results of the COMFORT-I and COMFORT-II Trials.[14]
In 2014, it was approved in polycythemia vera (PCV) when there has been an inadequate response to or intolerance of hydroxyurea, based on the RESPONSE trial.[15][5]
Research
It is also being investigated for plaque psoriasis,[6] alopecia areata,[16] relapsed diffuse large B-cell lymphoma, and peripheral T-cell lymphoma.[17]
In Feb 2016, a phase III trial for pancreatic cancer was terminated due to insufficient efficacy.[18]
Eight weeks-treatment with ruxolitinib blunted senescent cell-mediated inhibition of adipogenesis and increased insulin sensitivity in 22-month-old mice.[19]
As of September 2019. a clinical trial was in progress to evaluate "Treatment Free Remission After Combination Therapy With Ruxolitinib Plus Tyrosine Kinase Inhibitors".[20]
References
- "Jakafi (ruxolitinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 16 February 2014.
- Mesa RA, Yasothan U, Kirkpatrick P (February 2012). "Ruxolitinib". Nature Reviews. Drug Discovery. 11 (2): 103–4. doi:10.1038/nrd3652. PMID 22293561.
- Harrison C, Mesa R, Ross D, Mead A, Keohane C, Gotlib J, Verstovsek S (October 2013). "Practical management of patients with myelofibrosis receiving ruxolitinib". Expert Review of Hematology. 6 (5): 511–23. doi:10.1586/17474086.2013.827413. PMID 24083419.
- "Highlights of Prescribign Information: JAKAFI (ruxolitinib) tablets, for oral use" (PDF).
- Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. (January 2015). "Ruxolitinib versus standard therapy for the treatment of polycythemia vera". The New England Journal of Medicine. 372 (5): 426–35. doi:10.1056/NEJMoa1409002. PMC 4358820. PMID 25629741.
- Mesa RA (June 2010). "Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis". IDrugs. 13 (6): 394–403. PMID 20506062.
- Pardanani A, Tefferi A (March 2011). "Targeting myeloproliferative neoplasms with JAK inhibitors". Current Opinion in Hematology. 18 (2): 105–10. doi:10.1097/MOH.0b013e3283439964. PMID 21245760.
- Hobbs GS, Rampal RK (2015). "JAK2 Mutations and JAK Inhibitors in the Management of Myeloproliferative Neoplasms". Contemporary Oncology. 7 (1): 22–28.
- Wysham NG, Sullivan DR, Allada G (May 2013). "An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor". Chest. 143 (5): 1478–1479. doi:10.1378/chest.12-1604. PMC 5991580. PMID 23648912.
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. (March 2012). "JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis". The New England Journal of Medicine. 366 (9): 787–98. doi:10.1056/NEJMoa1110556. hdl:2158/605459. PMID 22375970.
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. (March 2012). "A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis". The New England Journal of Medicine. 366 (9): 799–807. doi:10.1056/NEJMoa1110557. PMC 4822164. PMID 22375971.
- Tefferi A (March 2012). "Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms". The New England Journal of Medicine. 366 (9): 844–6. doi:10.1056/NEJMe1115119. PMID 22375977.
- ASCO Annual Meeting 2011: JAK Inhibitor Ruxolitinib Demonstrates Significant Clinical Benefit in Myelofibrosis Archived November 21, 2011, at the Wayback Machine
- "FDA Approves Incyte's Jakafi (ruxolitinib) for Patients with Myelofibrosis" (Press release). Incyte. Retrieved 2012-01-02.
- Kaminskas E (4 December 2014). "Supplemental FDA approval letter for Jakafi (ruxolitinib) tablets" (PDF). U.S. Food and Drug Administration.
- Falto-Aizpurua L, Choudhary S, Tosti A (December 2014). "Emerging treatments in alopecia". Expert Opinion on Emerging Drugs. 19 (4): 545–56. doi:10.1517/14728214.2014.974550. PMID 25330928.
- Clinical trial number NCT01431209 for "Ruxolitinib Phosphate (Oral JAK Inhibitor INCB18424) in Treating Patients With Relapsed or Refractory Diffuse Large B-Cell or Peripheral T-Cell Non-Hodgkin Lymphoma" at ClinicalTrials.gov
- House DW (February 2016). "Incyte bags late-stage development of Jakafi for solid tumors; shares down 10% premarket". Seeking Alpha.
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. (December 2015). "Targeting senescent cells enhances adipogenesis and metabolic function in old age". eLife. 4: e12997. doi:10.7554/eLife.12997. PMC 4758946. PMID 26687007.
- Clinical trial number NCT03610971 for "Treatment Free Remission After Combination Therapy With Ruxolitinib Plus Tyrosine Kinase Inhibitors" at ClinicalTrials.gov