Norsalsolinol
Norsalsolinol is a chemical compound that is produced naturally in the body through metabolism of dopamine.[1] It has been shown to be a selective dopaminergic neurotoxin,[2][3][4] and has been suggested as a possible cause of neurodegenerative conditions such as Parkinson's disease and the brain damage associated with alcoholism,[5][6] although evidence for a causal relationship is unclear.[7][8][9]
 | |
 | |
| Names | |
|---|---|
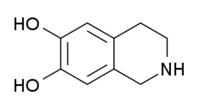
| IUPAC name
1,2,3,4-tetrahydroisoquinoline-6,7-diol | |
| Other names
6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11NO2 | |
| Molar mass | 165.189 g/mol |
| Hazards | |
| Main hazards | Neurotoxin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
(R)-Salsolinol which has been shown to be a product of ethanol metabolism, stereospecifically induces behavioral sensitization and leads to excessive alcohol intake in rats[10]
See also
References
- Maruyama W, Takahashi T, Minami M, Takahashi A, Dostert P, Nagatsu T, Naoi M (1993). "Cytotoxicity of dopamine-derived 6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolines". Advances in Neurology. 60: 224–30. PMID 8093579.
- Maruyama Y, Suzuki Y, Kazusaka A, Fujita S (May 2001). "Norsalsolinol uptake into secretory vesicles via vesicular monoamine transporter and its secretion by membrane depolarization or purinoceptor stimulation in PC12 cells". The Journal of Veterinary Medical Science. 63 (5): 493–7. doi:10.1292/jvms.63.493. PMID 11411492.
- Maruyama Y, Suzuki Y, Kazusaka A, Fujita S (June 2001). "Uptake of the dopaminergic neurotoxin, norsalsolinol, into PC12 cells via dopamine transporter". Archives of Toxicology. 75 (4): 209–13. doi:10.1007/s002040000202. PMID 11482518.
- Kobayashi H, Fukuhara K, Tada-Oikawa S, Yada Y, Hiraku Y, Murata M, Oikawa S (January 2009). "The mechanisms of oxidative DNA damage and apoptosis induced by norsalsolinol, an endogenous tetrahydroisoquinoline derivative associated with Parkinson's disease". Journal of Neurochemistry. 108 (2): 397–407. doi:10.1111/j.1471-4159.2008.05774.x. PMID 19012744.
- Dostert P, Strolin Benedetti M, Della Vedova F, Allievi C, La Croix R, Dordain G, Vernay D, Durif F (1993). "Dopamine-derived tetrahydroisoquinolines and Parkinson's disease". Advances in Neurology. 60: 218–23. PMID 8420138.
- Musshoff F, Daldrup T, Bonte W, Leitner A, Lesch OM (October 1997). "Salsolinol and norsalsolinol in human urine samples". Pharmacology Biochemistry and Behavior. 58 (2): 545–50. doi:10.1016/S0091-3057(97)00251-7. PMID 9300617.
- Musshoff F, Lachenmeier DW, Kroener L, Schmidt P, Dettmeyer R, Madea B (July 2003). "Simultaneous gas chromatographic-mass spectrometric determination of dopamine, norsalsolinol and salsolinol enantiomers in brain samples of a large human collective". Cellular and Molecular Biology (Noisy-le-Grand, France). 49 (5): 837–49. PMID 14528920.
- Scholz J, Klingemann I, Moser A (April 2004). "Increased systemic levels of norsalsolinol derivatives are induced by levodopa treatment and do not represent biological markers of Parkinson's disease". Journal of Neurology, Neurosurgery, and Psychiatry. 75 (4): 634–6. doi:10.1136/jnnp.2003.010769. PMC 1739023. PMID 15026514.
- Musshoff F, Lachenmeier DW, Schmidt P, Dettmeyer R, Madea B (January 2005). "Systematic regional study of dopamine, norsalsolinol, and (R/S)-salsolinol levels in human brain areas of alcoholics". Alcoholism: Clinical and Experimental Research. 29 (1): 46–52. doi:10.1097/01.ALC.0000150011.81102.C2. PMID 15654290.
- "(R)-Salsolinol, a product of ethanol metabolism, stereospecifically induces behavioral sensitization and leads to excessive alcohol intake. | PubFacts.com". www.pubfacts.com. Retrieved 2017-10-02.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.