Naloxazone
Naloxazone is an irreversible μ-opioid receptor antagonist which is selective for the μ1 receptor subtype.[1] Naloxazone produces very long lasting antagonist effects as it forms a covalent bond to the active site of the mu-opioid receptor,[2] thus making it impossible for the molecule to unbind and blocking the receptor permanently until the receptor is recycled by endocytosis.
 | |
| Names | |
|---|---|
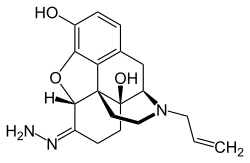
| IUPAC name
(5α)-17-Allyl-3,14-dihydroxy-4,5-epoxymorphinan-6-one hydrazone | |
| Other names
Naloxone- 6-hydrazone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
PubChem CID |
|
| |
| Properties | |
| C19H23N3O3 | |
| Molar mass | 341.40422 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It has been reported that naloxazone is unstable in acidic solution, dimerizing into the more stable and much more potent antagonist naloxonazine.[3] Under conditions in which no naloxonazine formation could be detected, naloxazone did not display irreversible μ opioid receptor binding.[3]
See also
- Chlornaltrexamine, an irreversible mixed agonist-antagonist
- Oxymorphazone, an irreversible μ-opioid full agonist
References
- Pasternak, G.; Childers; Snyder, S. (1980). "Opiate analgesia: Evidence for mediation by a subpopulation of opiate receptors". Science. 208 (4443): 514–6. Bibcode:1980Sci...208..514P. doi:10.1126/science.6245448. PMID 6245448.
- Ling, Geoffrey S.F.; Simantov, Ronit; Clark, Janet A.; Pasternak, Gavril W. (1986). "Naloxonazine actions in vivo". European Journal of Pharmacology. 129 (1–2): 33–8. doi:10.1016/0014-2999(86)90333-X. PMID 3021478.
- Hahn, Elliot F.; Pasternak, Gavril W. (1982). "Naloxonazine, a potent, long-lasting inhibitor of opiate binding sites". Life Sciences. 31 (12–13): 1385–8. doi:10.1016/0024-3205(82)90387-3. PMID 6292633.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.