Xylidine
Xylidine can refer to any of the six isomers of xylene amine, or any mixture of them.
The chemical formula of xylidines is C8H11N or, more descriptively, (CH3)2C6H3NH2. The CAS number for the isomer mixture is . They are colorless solids or liquids, although commercial samples can appear yellow or darker. They are miscible with ethanol and diethyl ether and slightly soluble in water. Xylidines are used in production of pigments and dyestuffs, and various antioxidants, agrochemicals, pharmaceuticals, hypergolic propellants, and many other organic chemicals.[1]
Isomers of xylidine
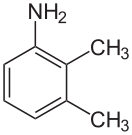 2,3-xylidine
2,3-xylidine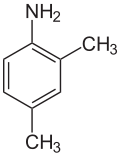 2,4-xylidine
2,4-xylidine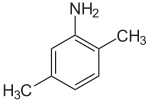 2,5-xylidine
2,5-xylidine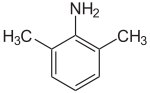 2,6-xylidine
2,6-xylidine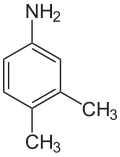 3,4-xylidine
3,4-xylidine 3,5-xylidine
3,5-xylidine
2,3-xylidine
2,3-xylidine, also called o-xylidine, 2,3-dimethylaniline, 2,3-xylylamine, or 2,3-dimethylphenylamine, is a liquid with melting point 2.5 °C and boiling point 222 °C, and flash point at 96 °C. Its CAS number is and its SMILES structure is Nc1cccc(C)c1C. It is used in production of mefenamic acid, dyes, and pesticides.
2,4-xylidine
2,4-xylidine, also called 2-methyl-p-toluidine, 2,4-dimethylaniline, 2,4-xylylamine, or 2,4-dimethylphenylamine, is a liquid with melting point 16 °C, boiling point 217 °C, and flash point at 90 °C. Its CAS number is and its SMILES structure is Nc1ccc(C)cc1C. It is used for production of pesticides, dyes, and other chemicals.
2,5-xylidine
2,5-xylidine, also called p-xylidine, 2,5-dimethylaniline, 2,5-xylylamine, or 2,5-dimethylphenylamine, is a liquid with melting point 11.5 °C and boiling point 215 °C. Its CAS number is and its SMILES structure is Nc1cc(C)ccc1C. It is used for production of dyes and other chemicals.
2,6-xylidine
2,6-Xylidine, also called 2,6-dimethylaniline, 2,6-xylylamine, or 2,6-dimethylphenylamine, is a liquid with melting point 8.4 °C and boiling point 216 °C. Its CAS number is and its SMILES structure is Nc1c(C)cccc1C. It is used in production of some anesthetics and other chemicals.
3,4-xylidine
3,4-xylidine, also called 3,4-dimethylaniline, 3,4-xylylamine, or 3,4-dimethylphenylamine, is a crystalline solid with melting point 51 °C and boiling point 226 °C. Its CAS number is and its SMILES structure is Nc1ccc(C)c(C)c1. It is used as a raw material for production of vitamin B2, dyes, pesticides, and other chemicals.
3,5-xylidine
3,5-xylidine, also called 3,5-dimethylaniline, 3,5-xylylamine, or 3,5-dimethylphenylamine, is a liquid with melting point 9.8 °C and boiling point 220–221 °C. Its CAS number is and its SMILES structure is Nc1cc(C)cc(C)c1. It is used for production of pesticides, dyes, and other chemicals.
Safety
The U.S. Occupational Safety and Health Administration (OSHA) has set the legal limit (Permissible exposure limit) for xylidine exposure in the workplace as 5 ppm (25 mg/m3) skin exposure over an eight-hour workday. The U.S. National Institute for Occupational Safety and Health (NIOSH) has set a Recommended exposure limit (REL) of 2 ppm (10 mg/m3) skin exposure over an eight-hour workday. At levels of 50 ppm, xylidine is immediately dangerous to life and health.[2]
Their risk and safety phrases are R20 R22 R36 R37 R38.
External links
References
- M. Meyer (2012). "Xylidines". Ullmann's Encylclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455.
- "CDC - NIOSH Pocket Guide to Chemical Hazards - Xylidine". www.cdc.gov. Retrieved 2015-11-28.