3,5-Xylidine
3,5-Xylidine is the organic compound with the formula C6H3(CH3)2NH2. it is one of several isomeric xylidines. It is a colorless viscous liquid. It is used in the production of the dye Pigment Red 149.[1]
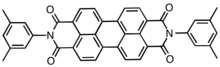
Chemical structure of Pigment Red 149
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.280 |
| EC Number |
|
| MeSH | C514328 |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.183 g·mol−1 |
| Appearance | colorless oil |
| Density | 0.9704 g/cm3 |
| Melting point | 9.8–10.0 °C (49.6–50.0 °F; 282.9–283.1 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| Flash point | 103 °C (217 °F; 376 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
3,5-Xylidine is produced industrially by amination of the xylenol using ammonia and alumina catalyst.
gollark: I must briefly leave, sorry.
gollark: I try a torso regrowth spell, d6.
gollark: Oh. Hmm.
gollark: Wait, while I'm in the crocodile, I grab the macguffin, d6.
gollark: I ask the crocodile to unconsume me, 2d6.
References
- M. Meyer (2012). "Xylidines". Ullmann's Encylclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.