Virtual breakdown mechanism
Virtual breakdown mechanism is a concept in the field of electrochemistry. In electrochemical reactions, when the cathode and the anode are close enough to each other (i.e., so-called "nanogap electrochemical cells"), the double layer regions from the two electrodes can be overlapped, forming large electric field uniformly distributed inside the entire electrode gap. Such high electric field can significantly enhance the ion migration inside bulk solution, and thus facilitate the entire reaction rate, performing like "breakdown" of the reactant(s). However, it is fundamentally different from the meaning of traditional "breakdown".
Virtual breakdown mechanism is discovered in 2017 when researchers studied pure water electrolysis based on deep-sub-Debye-length nanogap electrochemical cells. Furthermore, researchers found the size effect of the gap distance between cathodes and anodes to the performance of electrochemical reactions.[1]
Electric field distribution
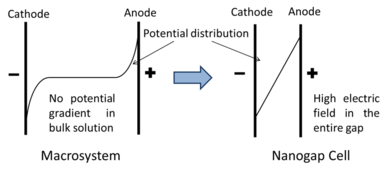
The fundamental difference between traditional cells and nanogap cells are their electric potential distribution. This is the premise of the "virtual breakdown" effect.
For electrochemical reactions with high-concentration electrolyte in macrosystem, the Debye-length is quite small. Due to the screening effect, almost all the potential drop is confined within such small Debye-length region (or double layer region). The potential in bulk solution (far from the electrodes) would not change too much, meaning that there is nearly zero electric field inside the bulk solution. However, when we put the counter electrode within the Debye-length region (i.e., nanogap electrochemical cells), two double layers from anode and cathode have to be overlapped with each other. The electrostatic potential inside the entire gap can change continuously and dramatically, meaning that huge electric field can be uniformly distributed in the entire gap.
Pure water electrolysis
Take pure water electrolysis as an example to explain the concept of Virtual breakdown mechanism.
Pure water electrolysis in macrosystem
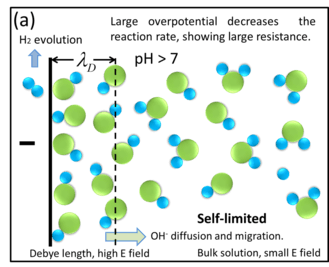
For water electrolysis, here use H3O+ ions and cathode as an example to explain the traditional reactions.
Water molecules can be self-ionized to H3O+ and OH− ions. Near the cathode surface (within double layer region), newly-generated H3O+ ions can become hydrogen gas after obtaining electrons from cathode ; However, because there is nearly no electric field inside bulk solution (see section "Electric field distribution"), such OH− ions can only transport through bulk solution very slowly by diffusion step. Moreover, in pure water the intrinsic H3O+ concentration is only 10−7 mol/L, not enough to neutralize the newly-generated OH− ions. In this way, OH− ions have to accumulate locally at the cathode surface (turning the solution near cathode into alkaline). Due to the Le Chatelier's principle, for water self-ionization,
the OH− ions accumulation can impede water further self-ionization, reducing the hydrogen evolution rate and eventually preventing water electrolysis. In this case, water electrolysis becomes very slow or even self-stopped, showing a large equivalent resistance between the two electrodes.
That is why in macrosystem pure water cannot be electrolyzed efficiently. The fundamental reason is the "lack of rapid ions transport inside bulk solution".[1]
Pure water electrolysis in nanogap cell
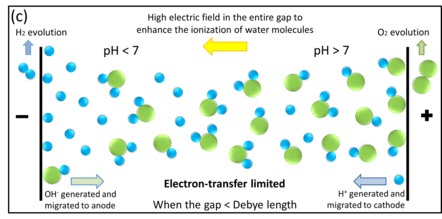
In nanogap cells, high electric field can distribute uniformly in the entire gap (see section "Electric field distribution"). Different from ion transport in macrosystem, now newly-generated OH− ions can be immediately migrated from cathode to anode. In the case where the two electrodes are close enough, the mass transport rate can be even larger than the electron-transfer rate, resulting in such OH− ions waiting for electron-transfer at the anode, rather than accumulated at the cathode. In this way, the entire reaction can keep going and no self-stopped.
Notice that, for pure water electrolysis in nanogap cells, the net OH− ion accumulation near the anode not only increases the local reactant concentration, but also decreases the overpotential requirement (as in the Frumkin effect).[2] According to Butler–Volmer equation, such ions accumulation can increase the electrolysis current, a.k.a., water splitting throughput and efficiency.
The traditional view should be revised that even pure water can be efficiently electrolyzed, when the electrode gap is small enough.
Virtual breakdown mechanism
In reality, water molecules dissociation (splitting into H3O+ and OH− ions) occurs only at the electrode region (because of the ions continuous consumed at the two electrodes); however, it appears like that the molecule split in the middle of the gap, with H3O+ ions migrating towards the cathode and OH− ions migrating towards the anode, respectively. With the help of huge electric field in the gap (see section "Electric field distribution"), not only the transport rate increases, but also the water molecules ionization has been enhanced (i.e., local concentration has been enhanced). From a microscopic perspective, the total effect looks like breakdown of water molecules.
However, this effect is not traditional breakdown, which in fact requires much larger electric field around 1 V/Å,.[3] In the nanogap cells, the huge electric field is still not large enough to split water molecules directly. However, "it can take advantage of the self-ionization of water (splitting into H3O+ and OH− ions, and those two types of ions are continuously consumed at the two electrodes), facilitating the equilibrium reaction to shift in the ionization direction"[1]
Such field-assisted ionization, with the fast ion transport (mainly migration), performs very similar to the breakdown of water molecules. That is why this field-assisted effect is named "virtual breakdown mechanism".
Consider the equation of conductivity,
Here the ion charges are not changed. The ion concentration is enhanced but only contributes to the conductivity partially. The fundamental change here is that "apparent mobility" has been significantly enhanced, as "breakdown" effect. (In traditional electrochemical cells, although the ion intrinsic mobility is high, since there is nearly zero electric field inside bulk solution, it cannot contribute to the conductivity.) Consider the equivalent resistance between the two electrodes, as given by
When we decrease the gap distance between the two electrodes, "not only the value of L decreases, but also the value of resistivity decreases as well, which in fact contributes more to the decrease of the total resistance".[1]
This "virtual breakdown mechanism" can be applied on almost all kinds of "weakly-ionized" materials, In fact, such weaker ionization can lead to larger Debye-length inside the solution. At the same size scale, it actually helps to achieve the virtual breakdown effect.
Gap size effect
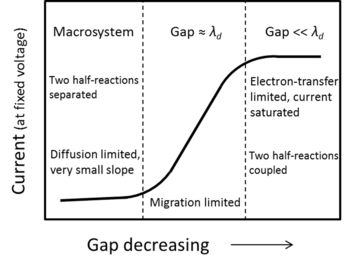
The phase diagram here shows how important of the electrode gap distance to the performance of electrochemical reactions. For traditional macrosystem where the electrode gap distance is much larger than the Debye-length, two half-reactions are decoupled and cannot feel each other. Normally the electrochemical current is limited by slow diffusion step. When gap distance has been reduced to around Debye-length, large electric field can form between the two electrodes (due to double layers from two electrodes overlapping with each other) to enhance the mass transport rate. In this region, the electrolysis current is very sensitive to the gap distance and the reactions are migration limited. When the gap distance is further reduced to deep-sub-Debye-length region, the mass transport can be enhanced further to the level even faster than electron-transfer step. In this region, even we shrink the gap distance further the current cannot be enlarged any more, meaning that the current has reached saturation. Here the two half-reactions are coupled together and the reactions are limited by electron-transfer steps.
By just adjusting the gap distance, the fundamental performance of the electrochemical reactions can be significantly changed.
References
- Wang, Yifei; Narayanan, S. R.; Wu, Wei (2017-07-11). "Field-Assisted Splitting of Pure Water Based on Deep-Sub-Debye-Length Nanogap Electrochemical Cells". ACS Nano. 11 (8): 8421–8428. doi:10.1021/acsnano.7b04038. ISSN 1936-0851. PMID 28686412.
- De Kreuk, C.W.; Sluyters-Rehbach, M.; Sluyters, J.H. (December 1970). "Electrode kinetics and double-layer structure". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 28 (2): 391–407. doi:10.1016/s0022-0728(70)80133-4. hdl:1874/15071. ISSN 0022-0728.
- Stuve, Eric M. (January 2012). "Ionization of water in interfacial electric fields: An electrochemical view". Chemical Physics Letters. 519-520: 1–17. doi:10.1016/j.cplett.2011.09.040. ISSN 0009-2614.