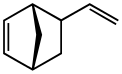
Vinyl norbornene
Vinyl norbornene (VNB) is an organic compound that consists of a vinyl group attached to norbornene. It is a colorless liquid. The compound exists as endo and exo isomers, but these are not typically separated. It is an intermediate in the production of the commercial polymer EPDM. It is prepared by the Diels-Alder reaction of butadiene and cyclopentadiene.[1]
Vinyl norbornene is an intermediate in the production ethylidene norbornene.
 | |
| Names | |
|---|---|
| IUPAC name
5-Ethenylbicyclo[2.2.1]hept-2-ene | |
| Other names
2-Ethylidene-5-norbornene; 5-Vinyl-2-norbornene | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C9H12 | |
| Molar mass | 120.195 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 141 °C (286 °F; 414 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Safety
LD50 (intravenous, rabbit = 0.10–0.05 mg/kg(female). It is also a neurotoxin.[2]
gollark: This is fearsome, yes.
gollark: https://www.pnas.org/content/119/8/e2120481119
gollark: Well, yes.
gollark: You did, yes.
gollark: Maybe I should add baidicoot at some point. Oh well.
References
- Behr, Arno (2000). "Organometallic Compounds and Homogeneous Catalysis". Ullmann's Encyclopedia of Industrial Chemistry. p. 10. doi:10.1002/14356007.a18_215. ISBN 978-3527306732.
- Ballantyne, Bryan; Myers, Roy C.; Klonne, Dennis R. (1997). "Comparative acute toxicity and primary irritancy of the ethylidene and vinyl isomers of norbornene". Journal of Applied Toxicology. 17 (4): 211–221. doi:10.1002/(SICI)1099-1263(199707)17:4<211::AID-JAT430>3.0.CO;2-X.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.