Ethylidene norbornene
Ethylidene norbornene (ENB) is an organic compound that consists of an ethylene group attached to norbornene. It is a colorless liquid. The molecule consists of two sites of unsaturation, one of which participates in the copolymerization and the second of which (the ethylidene) undergoes vulcanization. It is a monomer in the production of the commercial polymer EPDM. The compound consists of E- and Z-stereoisomers, but the mixtures are not separated.
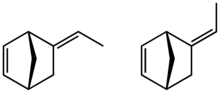 | |
| Names | |
|---|---|
| Other names
2-ethylidene-5-norbornene | |
| Identifiers | |
PubChem CID |
|
| UNII | |
| Properties | |
| C9H12 | |
| Molar mass | 120.195 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.893 g/mL |
| Melting point | −80 °C (−112 °F; 193 K) |
| Boiling point | 146 °C (295 °F; 419 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is prepared by isomerization of vinyl norbornene, which in turn is obtained by the Diels-Alder reaction of butadiene and cyclopentadiene.[1]
Ethylidene norbornene can be produced in two steps from cyclopentadiene.
Safety
Its LD50 (intravenous, rabbit) ranges from 0.09 (male rabbit) to 0.11 ml/kg (female). It is also a neurotoxin.[2]
gollark: I "would" use APL if it could be typed and also if there was a good FOSS implementation.
gollark: It does it with magic async stuff internally I believe.
gollark: Well, as much as I generally dislike Go, it gets it somewhat right: everything is asynchronous anyway, and you have green threads, so no magic sprinkling of `async` everywhere.
gollark: It's ENTIRELY spoilers?
gollark: ++magic py (await bot.get_channel(412764872816852994).fetch_message(785111865402720256)).content.replace("|", "¦")
References
- Behr, Arno (2000). "Organometallic Compounds and Homogeneous Catalysis". Ullmann's Encyclopedia of Industrial Chemistry. p. 10. doi:10.1002/14356007.a18_215.
- Ballantyne, Bryan; Myers, Roy C.; Klonne, Dennis R. (1997). "Comparative acute toxicity and primary irritancy of the ethylidene and vinyl isomers of norbornene". Journal of Applied Toxicology. 17: 211–221. doi:10.1002/(SICI)1099-1263(199707)17:4<211::AID-JAT430>3.0.CO;2-X.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.