Vinyl bromide
Vinyl bromide is a simple vinyl halide. It is a colorless liquid. It is produced from ethylene dibromide. It is mainly used as a comonomer to confer fire retardant properties to acrylate polymers.[2]
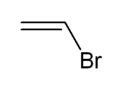 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bromoethene | |
| Other names
Vinyl bromide 1-Bromoethene Bromoethylene 1-Bromoethylene Monobromoethene Monobromoethylene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.911 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3Br | |
| Molar mass | 106.95 g/mol |
| Appearance | Colorless gas |
| Odor | pleasant[1] |
| Density | 1.525 g/cm3 at boiling point (liquid)
1.4933 g/cm3 at 20 °C |
| Melting point | −137.8 °C (−216.0 °F; 135.3 K) |
| Boiling point | 15.8 °C (60.4 °F; 288.9 K) |
| Insoluble | |
| log P | 1.57 |
| Vapor pressure | 206.8 kPa at 37.8 °C |
| Hazards | |
| Main hazards | Toxic (T), Highly flammable (F+) |
| Safety data sheet | See: data page |
| R-phrases (outdated) | R12, R20/21/22, R36/37/38, R45 |
| S-phrases (outdated) | S45, S53 |
| NFPA 704 (fire diamond) | |
| Flash point | 5 °C (41 °F; 278 K) |
| 530 °C (986 °F; 803 K) | |
| Explosive limits | 9%-15%[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
N.D.[1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions and applications
It reacts with magnesium to give the corresponding Grignard reagent.[3]
Safety precautions
Vinyl bromide is listed in List of IARC Group 2A carcinogens as a suspected human carcinogen.
gollark: <@!202992030685724675> Basically, the *rest* of the BIOS either finds the HDD saved in the EEPROM's memory (`computer.setBootAddress`/`getBootAddress` as defined elsewhere in it), or, if it's invalid or there is no boot address set for it, checks all connected disks.
gollark: I could add some fun "potatOS system dump" mode, yes.
gollark: Ah, of course.
gollark: `string.dump(string.dump)`
gollark: This is ***evil*** code.
See also
References
- NIOSH Pocket Guide to Chemical Hazards. "#0657". National Institute for Occupational Safety and Health (NIOSH).
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- Dietmar Seyferth (1959). "Di-n-butyldivinyltin". Org. Synth. 39: 10. doi:10.15227/orgsyn.039.0010.
External links
- International Chemical Safety Card 0597
- NIOSH Pocket Guide to Chemical Hazards. "#0657". National Institute for Occupational Safety and Health (NIOSH).
- MSDS at Oxford University
- MSDS at mathesontrigas.com
- Vinyl bromide at IRIS
- Vinyl bromide at osha.gov
- IARC Summary & Evaluation of vinyl bromide
- Report on Carcinogens Background Document for Vinyl Bromide
- Synthesis of vinyl bromides
- The Kinetics of Pyrolysis of Vinyl Bromide
- UV absorption spectra
- UV Spectrum and Cross Sections
- 1H NMR spectrum
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
