Twister ribozyme
The twister ribozyme[1] is a catalytic RNA structure capable of self-cleavage. The nucleolytic activity of this ribozyme has been demonstrated both in vivo and in vitro and has one of the fastest catalytic rates of naturally occurring ribozymes with similar function.[2][3] The twister ribozyme is considered to be a member of the small self-cleaving ribozyme family which includes the hammerhead, hairpin, hepatitis delta virus (HDV), Varkud satellite (VS), and glmS ribozymes.[3]
Discovery
In contrast to in vitro selection methods, which have aided in identifying several classes of catalytic RNA motifs, the twister ribozyme was discovered by a bioinformatics approach as a conserved RNA structure of unknown function.[1] The hypothesis that it functions as a self-cleaving ribozyme was suggested by the similarity between genes nearby to twister ribozymes and genes nearby to hammerhead ribozymes,[4] Indeed the genes located nearby to these two self-cleaving ribozyme classes overlap significantly.[1] Researchers were inspired to name the newly found twister motif due to its resemblance to the Egyptian hieroglyph 'twisted flax'.[1]
Structure
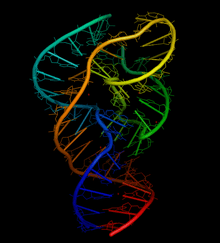
The basic structure of the Oryza sativa twister ribozyme was crystallographically determined at atomic resolution in 2014.[2] The active site of the twister ribozyme is centered in a double-pseudoknot, facilitating a compact fold structure through two long-range tertiary interactions, in partnership with a helical junction.[2] Magnesium is important for secondary structure stabilization of the ribozyme.[3]
Catalytic Mechanism
Similar to other nucleolytic ribozymes, the twister ribozyme selectively cleaves phopshodiester bonds, through an SN2-related mechanism, into a 2',3'-cyclic phosphate and 5' hydroxyl product.[1] Both experimental and modelling evidence have supported a concerted general-acid-base catalysis involving highly conserved adenine (A1) and guanine (G33) bases, where N3 of A1 acts as a proton donor and G33 the general base.[5][6][2] The twister ribozyme generates catalytic activity by specifically orienting the to-be-cleaved P O bond for in-line nucleophilic attack within the active site.[7] Currently, it is known that the rate of reaction of the twister ribozyme is dependent on both pH and temperature.[7][1] Replacements of the pro-S nonbridging oxygen of the scissile phosphate with a thiol group leads to reduced self-cleavage rates, suggesting that the mechanism is not reliant on bound magnesium. Rescue of the thiol-derivative by cadmium cations indicates that divalent metal ions play a role in rate enhancement.[6] A likely mechanism for this is the stabilization of the transition state by reducing electrostatic strain on the substrate strand from the growing negative charge during cleavage.
Prevalence in Nature
The twister ribozyme motif is relatively common in nature with 2,700 examples observed across bacteria, fungi, plants, and animals.[2] Similarly to hammerhead ribozymes, some eukaryotes contain large numbers of twister ribozymes. In the most extreme known example, there are 1051 predicted twister ribozymes in Schistosoma mansoni, an organism that also contains many hammerhead ribozymes. In bacteria, twister ribozymes are near to gene classes that are also commonly associated with bacterial hammerhead ribozymes. Currently, there is no understood biological function associated with the twister ribozyme.[7]
References
- Roth A, Weinberg Z, Chen AG, Kim PB, Ames TD, Breaker RR (2013). "A widespread self-cleaving ribozyme class is revealed by bioinformatics". Nat Chem Biol. 10 (1): 56–60. doi:10.1038/nchembio.1386. PMC 3867598. PMID 24240507.
- Liu Y, Wilson TJ, McPhee SA, Lilley DM (2014). "Crystal structure and mechanistic investigation of the twister ribozyme". Nat Chem Biol. 10 (9): 739–744. doi:10.1038/nchembio.1587. PMID 25038788.
- Eiler, Daniel; Wang, Jimin; Steitz, Thomas A. (2014-09-09). "Structural basis for the fast self-cleavage reaction catalyzed by the twister ribozyme". Proceedings of the National Academy of Sciences. 111 (36): 13028–13033. doi:10.1073/pnas.1414571111. ISSN 0027-8424. PMC 4246988. PMID 25157168.
- Perreault, Jonathan; Weinberg, Zasha; Roth, Adam; Popescu, Olivia; Chartrand, Pascal; Ferbeyre, Gerardo; Breaker, Ronald R. (2011-05-05). "Identification of Hammerhead Ribozymes in All Domains of Life Reveals Novel Structural Variations". PLOS Computational Biology. 7 (5): e1002031. doi:10.1371/journal.pcbi.1002031. ISSN 1553-7358. PMC 3088659. PMID 21573207.
- Gaines, Colin S.; York, Darrin M. (2016-03-09). "Ribozyme Catalysis with a Twist: Active State of the Twister Ribozyme in Solution Predicted from Molecular Simulation". Journal of the American Chemical Society. 138 (9): 3058–3065. doi:10.1021/jacs.5b12061. ISSN 0002-7863. PMC 4904722. PMID 26859432.
- Wilson, Timothy J.; Liu, Yijin; Domnick, Christof; Kath-Schorr, Stephanie; Lilley, David M. J. (2016-05-18). "The Novel Chemical Mechanism of the Twister Ribozyme". Journal of the American Chemical Society. 138 (19): 6151–6162. doi:10.1021/jacs.5b11791. ISSN 0002-7863. PMID 27153229.
- Gebetsberger, Jennifer; Micura, Ronald (2017-05-01). "Unwinding the twister ribozyme: from structure to mechanism". Wiley Interdisciplinary Reviews: RNA. 8 (3): n/a. doi:10.1002/wrna.1402. ISSN 1757-7012. PMC 5408937. PMID 27863022.