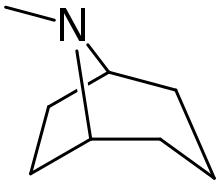
Tropane alkaloid
Tropane alkaloids are a class of bicyclic [3.2.1] alkaloids and secondary metabolites that contain a tropane ring in their chemical structure.[1] Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Some tropane alkaloids have pharmacological properties and can act as anticholinergics or stimulants.
Classification
Anticholinergics
Anticholinergic drugs[2] and deliriants:
- Atropine, racemic hyoscyamine, from the deadly nightshade (Atropa belladonna)
- Hyoscyamine, the levo-isomer of atropine, from henbane (Hyoscyamus niger), mandrake (Mandragora officinarum) and the sorcerers' tree (Latua pubiflora).
- Scopolamine, from henbane and Datura species (Jimson weed)
All three acetylcholine-inhibiting chemicals can also be found in the leaves, stems, and flowers in varying, unknown amounts in Brugmansia (angel trumpets), a relative of Datura. The same is also true of many other plants belonging to subfamily Solanoideae of the Solanaceae, the alkaloids being concentrated particularly in the leaves and seeds. However, the concentration of alkaloids can vary greatly, even from leaf to leaf and seed to seed.[3][4]
Stimulants
Stimulants and cocaine-related alkaloids:
- Cocaine, from coca plant (Erythroxylum coca)
- Ecgonine, a precursor and metabolite of cocaine
- Benzoylecgonine, a metabolite of cocaine
- Hydroxytropacocaine, from coca plant (Erythroxylum coca)
- Methylecgonine cinnamate, from coca plant (Erythroxylum coca)
Others
- Catuabines, found in catuaba, an infusion or dry extract made from Erythroxylum vaccinifolium
- Scopine
Synthetic analogs of tropane alkaloids also exist, such as the phenyltropanes. They are not considered to be alkaloids per definition.
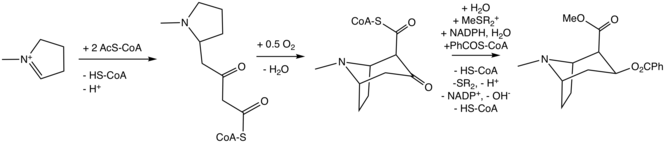
Biosynthesis
The biosynthesis of the tropane alkaloids have attracted intense interest because of their high physiological activity as well as the presence of the bicyclic tropane core.[5]
References
- O’Hagan, David (2000). "Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids (1998 to 1999)". Natural Product Reports. 17 (5): 435–446. doi:10.1039/a707613d.
- Grynkiewicz, G; Gadzikowska, M. "Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs". Pharmacological reports : PR. 60 (4): 439–63. PMID 18799813.
- The Biology and Taxonomy of the Solanaceae edited by Hawkes, J.G., Lester, R.N. and Skelding, A.D. (Linnean Society Symposium Series Number 7) Published for the Linnean Society of London by Academic Press 1979 ISBN 0-12-333150-1
- Eich, Prof. Dr. Eckhart, 2008, Solanaceae and Convolvulaceae : Secondary Metabolites - Biosynthesis, Chemotaxonomy, Biological and Economic Significance (A Handbook) pub. Springer-Verlag Berlin Heidelberg, ISBN 978-3-540-74541-9.
- Leete, Edward (1990). "Recent Developments in the Biosynthesis of the Tropane Alkaloids1". Planta Medica. 56 (4): 339–352. doi:10.1055/s-2006-960979. PMID 2236285.