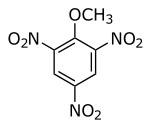
Trinitroanisole
Trinitroanisole is a chemical compound that exists as pale yellow crystals with a melting point of 68 °C. It is an explosive with a detonation velocity of 7200 meters per second.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-Methoxy-1,3,5-trinitrobenzene | |
| Other names
2,4,6-Trinitroanisol; picric acid methyl ether; trisol; trinol; trinitroanisole | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.149.212 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5N3O7 | |
| Molar mass | 243.131 g·mol−1 |
| Appearance | yellow, "leaf-like" crystals |
| Density | 1.61 g/cm3 |
| Melting point | 68 °C (154 °F; 341 K) |
| Boiling point | explodes |
| insoluble in water, soluble in diethyl ether and hot ethanol | |
| Hazards | |
| Main hazards | explosive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Trinitroanisole can be prepared by the reaction of 2,4-dinitrochlorobenzene with methanol in the presence of sodium hydroxide followed by the nitration of the resulting product. Alternatively, it can be prepared directly by the reaction of picryl chloride with methanol in the presence of sodium hydroxide.[1]
Use
Historically, trinitroanisole was used as a military explosive (e.g., Japanese Type 91), however, due to its tendency to form picric acid and dangerous picrate salts, its use has largely been abandoned.
Notes
- Wasag-Chemie, Essen. "Explosivstoffe". 1961, p. 164.
gollark: My Alpine-running system is upgrading impressively speedily.
gollark: *is suddenly reminded to run updates*
gollark: <@!202992030685724675> Basically, the *rest* of the BIOS either finds the HDD saved in the EEPROM's memory (`computer.setBootAddress`/`getBootAddress` as defined elsewhere in it), or, if it's invalid or there is no boot address set for it, checks all connected disks.
gollark: I could add some fun "potatOS system dump" mode, yes.
gollark: Ah, of course.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.