Trifluoroacetic acid
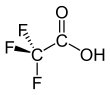
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group’s hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant that is approximately 34,000 times higher,[3] as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes.
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trifluoroacetic acid | |||
| Other names
2,2,2-Trifluoroacetic acid 2,2,2-Trifluoroethanoic acid Perfluoroacetic acid Trifluoroethanoic acid TFA | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.846 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2HF3O2 | |||
| Molar mass | 114.023 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | Pungent/Vinegar | ||
| Density | 1.489 g/cm3, 20 °C | ||
| Melting point | −15.4 °C (4.3 °F; 257.8 K) | ||
| Boiling point | 72.4 °C (162.3 °F; 345.5 K) | ||
| miscible | |||
| Vapor pressure | 0.0117 bar (1.17 kPa) at 20 °C[1] | ||
| Acidity (pKa) | 0.52 [2] | ||
| -43.3·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Highly corrosive | ||
| Safety data sheet | External MSDS | ||
| R-phrases (outdated) | R20 R35 R52/53 | ||
| S-phrases (outdated) | S9 S26 S27 S28 S45 S61 | ||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related perfluorinated acids |
Perfluorooctanoic acid Perfluorononanoic acid | ||
Related compounds |
Acetic acid Trichloroacetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
TFA is prepared industrially by the electrofluorination of acetyl chloride or acetic anhydride, followed by hydrolysis of the resulting trifluoroacetyl fluoride:[4]
- CH
3COCl + 4 HF → CF
3COF + 3 H
2 + HCl - CF
3COF + H
2O → CF
3COOH + HF
Where desired, this compound may be dried by addition of trifluoroacetic anhydride.[5]
An older route to TFA proceeds via the oxidation of 1,1,1-trifluoro-2,3,3-trichloropropene with potassium permanganate. The trifluorotrichloropropene can be prepared by Swarts fluorination of hexachloropropene.
TFA occurs naturally in sea water, but only in small concentrations (≤200 ng/L).[6][7]
Uses
TFA is the precursor to many other fluorinated compounds such as trifluoroacetic anhydride, trifluoroperacetic acid, and 2,2,2-trifluoroethanol.[4] It is a reagent used in organic synthesis because of a combination of convenient properties: volatility, solubility in organic solvents, and its strength as an acid.[8] TFA is also less oxidizing than sulfuric acid but more readily available in anhydrous form than many other acids. One complication to its use is that TFA forms an azeotrope with water (b. p. 105 °C).
TFA is popularly used as a strong acid to remove t-butyl derived side-chain protecting groups in Fmoc peptide synthesis, and in other organic syntheses to remove the t-butoxycarbonyl protecting group.[9][10]
At a low concentration, TFA is used as an ion pairing agent in liquid chromatography (HPLC) of organic compounds, particularly peptides and small proteins. TFA is a versatile solvent for NMR spectroscopy (for materials stable in acid). It is also used as a calibrant in mass spectrometry.[11]
TFA is used to produce trifluoroacetate salts.[12]
Implication in anaesthesia
TFA is a metabolic breakdown product of the volatile anaesthetic agent halothane. It is thought to be responsible for halothane induced hepatitis.
Safety
Trifluoroacetic acid is a corrosive acid but it does not pose the hazards associated with hydrofluoric acid because the carbon-fluorine bond is not labile. Only if heated or treated with ultrasonic waves will it decompose into hydrofluoric acid. TFA is harmful when inhaled, causes severe skin burns and is toxic for aquatic organisms even at low concentrations.
TFA's reaction with bases and metals, especially light metals, is strongly exothermic. The reaction with lithium aluminium hydride (LAH) results in an explosion.[13]
Environment
TFA is not readily biodegradable and is toxic to aquatic life - although it does not bioaccumulate, prevention of release into waterways is of extreme importance when using TFA.[14]
See also
- Fluoroacetic acid - Highly toxic but naturally occurring rodenticide CH2FCOOH
- Difluoroacetic acid
- Trichloroacetic acid, the chlorinated analog.
References
- "Trifluoroacetic acid". Welcome to the NIST WebBook. Retrieved 2020-03-01.
- W. M. Haynes.; David R. Lide; Thomas J. Bruno, eds. (2016–2017). CRC Handbook of Chemistry and Physics. pp. 954–963. ISBN 978-1-4987-5429-3.
- Note: Calculated from the ratio of the Ka values for TFA (pKa = 0.23) and acetic acid (pKa = 4.76)
- G. Siegemund; W. Schwertfeger; A. Feiring; B. Smart; F. Behr; H. Vogel; B. McKusick. "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- Wilfred L.F. Armarego & Christina Li Lin Chai. "Chapter 4 - Purification of Organic Chemicals". Purification of Laboratory Chemicals (6th ed.). doi:10.1016/B978-1-85617-567-8.50012-3.
- "Trifluoroacetate in ocean waters". Environ. Sci. Technol. 36 (1): 12–5. January 2002. Bibcode:2002EnST...36...12P. doi:10.1021/es0221659. PMID 11811478.
- "Trifluoroacetate profiles in the Arctic, Atlantic, and Pacific Oceans". Environ. Sci. Technol. 39 (17): 6555–60. September 2005. Bibcode:2005EnST...39.6555S. doi:10.1021/es047975u. PMID 16190212.
- Eidman, K. F.; Nichols, P. J. (2004). "Trifluoroacetic Acid". In L. Paquette (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289.
- Lundt, Behrend F.; Johansen, Nils L.; Vølund, Aage; Markussen, Jan (1978). "Removal of t-Butyl and t-Butoxycarbonyl Protecting Groups with Trifluoroacetic acid". International Journal of Peptide and Protein Research. 12 (5): 258–268. doi:10.1111/j.1399-3011.1978.tb02896.x. PMID 744685.
- Andrew B. Hughes. "1. Protection Reactions". In Vommina V. Sureshbabu; Narasimhamurthy Narendra (eds.). Amino Acids, Peptides and Proteins in Organic Chemistry: Protection Reactions, Medicinal Chemistry, Combinatorial Synthesis. 4. doi:10.1002/9783527631827.ch1.
- Stout, Steven J.; Dacunha, Adrian R. (1989). "Tuning and calibration in thermospray liquid chromatography/mass spectrometry using trifluoroacetic acid cluster ions". Analytical Chemistry. 61 (18): 2126. doi:10.1021/ac00193a027.
- O. Castano; A. Cavallaro; A. Palau; J. C. Gonzalez; M. Rossell; T. Puig; F. Sandiumenge; N. Mestres; S. Pinol; A. Pomar & X. Obradors (2003). "High quality YBa2Cu3O7 thin films grown by trifluoroacetates metal-organic deposition". Superconductor Science and Technology. 16 (1): 45–53. Bibcode:2003SuScT..16...45C. doi:10.1088/0953-2048/16/1/309.
- Safety data sheet for Trifluoroacetic acid (PDF) from EMD Millipore, revision date 10/27/2014.
- "GPS Safety Summary: Trifluoroacetic Acid" (PDF). Retrieved October 18, 2016.