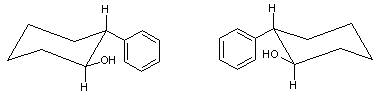
trans-2-Phenyl-1-cyclohexanol
trans-2-Phenyl-1-cyclohexanol is an organic compound. The two enantiomers of this compound are used in organic chemistry as chiral auxiliaries.
-2-phenylcyclohexanol-2D-skeletal.png) (1S,2R)-2-Phenylcyclohexanol | |
| Names | |
|---|---|
| Other names
trans-2-Phenylcyclohexanol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H16O | |
| Molar mass | 176.259 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
The enantioselective synthesis was accomplished by J. K. Whitesell by adding Pseudomonas fluorescens lipase to racemic trans-2-phenylcyclohexyl chloroacetate.[1][2] This enzyme is able to hydrolyze the ester bond of the (−)-enantiomer but not the (+)-enantiomer. The (−)-cyclohexanol and the (+)-ester are separated by fractional crystallization and the isolated (+)-ester hydrolyzed to the (+)-cyclohexanol in a separate step.
The enantiomers have also been prepared by the Sharpless asymmetric dihydroxylation of 1-phenylcyclohexene to the diol followed by the selective reduction of the 1-hydroxyl group by Raney nickel.[3]
References
- J. K. Whitesell, H. H. Chen and R. M. Lawrence (1985). "trans-2-Phenylcyclohexanol. A powerful and readily available chiral auxiliary". J. Org. Chem. 50 (23): 4663–4664. doi:10.1021/jo00223a055.
- A. Schwartz, P. Madan, J. K. Whitesell, and R. M. Lawrence (1993). "Lipase-Catalyzed Kinetic Resolution of Alcohols via Chloroacetate Esters: (−)-(1R,2S)-Trans-2-Phenylcyclohexanol And (+)-(1S,2R)-Trans-2-Phenylcyclohexanol". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 8, p. 516
- Javier Gonzalez, Christine Aurigemma, and Larry Truesdale (2004). "Sharpless bishydroxylation procedure to trans-2-phenyl-1-cyclohexanol". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 603