Total inorganic carbon
Total inorganic carbon (CT or TIC) is the sum of inorganic carbon species.
Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, HCO3−, CO32− respectively).
Inorganic carbon species
The inorganic carbon species include carbon dioxide, carbonic acid, bicarbonate anion, and carbonate.[1] It is customary to express carbon dioxide and carbonic acid simultaneously as CO2* . CT is a key parameter when making measurements related to the pH of natural aqueous systems,[2] and carbon dioxide flux estimates.
- CT = [CO2*] + [HCO3−] + [CO32−]
where,
- CT is the total inorganic carbon
- [CO2*] is the sum of carbon dioxide and carbonic acid concentrations ( [CO2*] = [CO2] + [H2CO3])
- [HCO3−] is the bicarbonate concentration
- [CO32−] is the carbonate concentration
Each of these species are related by the following pH-driven chemical equilibria:
- CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− ⇌ 2H+ + CO32−
The concentrations of the different species of DIC (and which species is dominant) depends on the pH of the solution, as shown by a Bjerrum plot.
Total inorganic carbon is typically measured by the acidification of the sample which drives the equilibria to CO2. This gas is then sparged from solution and trapped, and the quantity trapped is then measured, usually by infrared spectroscopy.
Marine carbon
.svg.png)
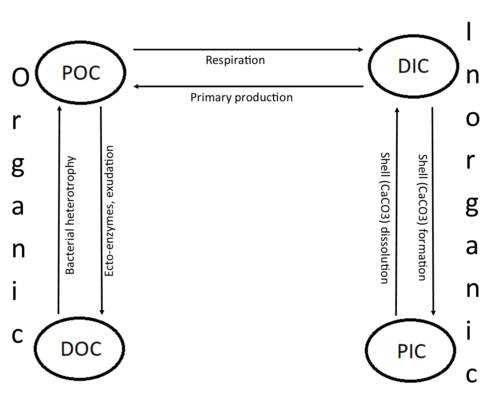
Marine carbon is further separated into particulate and dissolved phases. These pools are operationally defined by physical separation – dissolved carbon passes through a 0.2 μm filter, and particulate carbon does not.
There are two main types of inorganic carbon that are found in the oceans:
- Dissolved inorganic carbon (DIC) is made up of bicarbonate (HCO3−), carbonate (CO32−) and carbon dioxide (including both dissolved CO2 and carbonic acid H2CO3). DIC can be converted to particulate inorganic carbon (PIC) through precipitation of CaCO3 (biologically or abiotically). DIC can also be converted to particulate organic carbon (POC) through photosynthesis and chemoautotrophy (i.e. primary production). DIC increases with depth as organic carbon particles sink and are respired. Free oxygen decreases as DIC increases because oxygen is consumed during aerobic respiration.
- Particulate inorganic carbon (PIC) is the other form of inorganic carbon found in the ocean. Most PIC is the CaCO3 that makes up shells of various marine organisms, but can also form in whiting events. Marine fish also excrete calcium carbonate during osmoregulation.[3]
Some of the inorganic carbon species in the ocean, such as bicarbonate and carbonate, are major contributors to alkalinity, a natural ocean buffer that prevents drastic changes in acidity (or pH). The marine carbon cycle also affects the reaction and dissolution rates of some chemical compounds, regulates the amount of carbon dioxide in the atmosphere and Earth's temperature.[4]
References
- C. Michael Hogan. 2010. Calcium. eds. A. Jorgensen, C. Cleveland. Encyclopedia of Earth. National Council for Science and the Environment.
- Stanley E. Manahan. 2005. Environmental chemistry. CRC Press
- Wilson, R. W.; Millero, F. J.; Taylor, J. R.; Walsh, P. J.; Christensen, V.; Jennings, S.; Grosell, M. (2009-01-16). "Contribution of Fish to the Marine Inorganic Carbon Cycle". Science. 323 (5912): 359–362. Bibcode:2009Sci...323..359W. doi:10.1126/science.1157972. ISSN 0036-8075. PMID 19150840.
- Emerson, Steven (2008). Chemical Oceanography and the Marine Carbon Cycle. United Kingdom: Cambridge University Press. ISBN 978-0-521-83313-4.