Dissolved inorganic carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids.
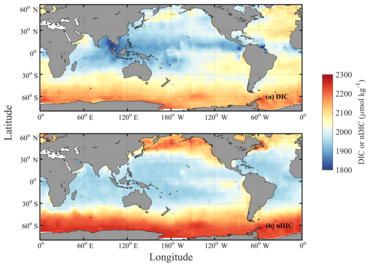
Spatial distribution of ocean surface DIC [1]
Spatial distributions of DIC and nDIC. (a) DIC (normalized to year 2005); (b) salinity-normalized DIC (nDIC, DIC normalized to reference year of 2005 and salinity of 35) in the surface global ocean. The latitudinal trends are clear, particularly for nDIC.Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, HCO3−, CO32− respectively). Dissolved inorganic carbon (DIC) includes three major aqueous species, CO2, HCO3- ,CO32−, and to a lesser extent their complexes in solution with metal ions.[2]
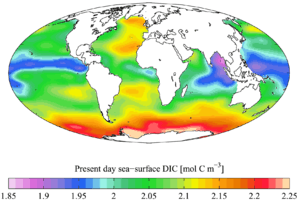
"Present day" (1990s)
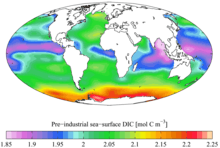
Pre-industrial (1700s)
See also
- Alkalinity (total alkalinity; AT)
- Bjerrum plot
- Dissolved organic carbon
- Fugacity (carbon dioxide fugacity; fCO2)
- Ocean acidification
- pH
- Total organic carbon
References
- Wu, Y., Hain, M.P., Humphreys, M.P., Hartman, S. and Tyrrell, T. (2019) "What drives the latitudinal gradient in open-ocean surface dissolved inorganic carbon concentration?" Biogeosciences, 16(13): 2661–2681. doi:10.5194/bg-16-2661-2019.

- Mackenzie FT and Lerman A (2006) Carbon in the Geobiosphere: Earth's Outer Shell, Springer Science & Business Media. ISBN 9781402042386.
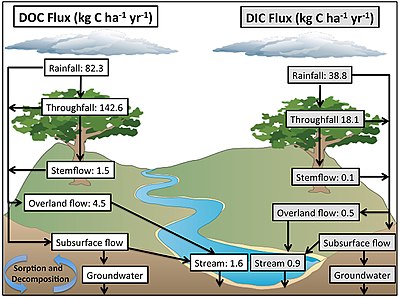
- Environmental Research at Tanguro Ranch, Brazil Esri. Retrieved 26 July 2020.
- Neu, V., Ward, N.D., Krusche, A.V. and Nill, C. (2016) "Dissolved organic and inorganic carbon flow paths in an Amazonian transitional forest". Frontiers in Marine Science, 3: 114. doi:10.3389/fmars.2016.00114.

This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.