Thiocarbanilide
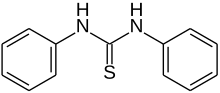
Thiocarbanilide is an organic chemical compound with the formula (C6H5NH)2CS. This white solid is a derivative of thiourea. It is prepared by the reaction of aniline and carbon disulfide.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N'-Diphenylthiourea | |
| Other names
1,3-Diphenylthiourea sym-Diphenylthiourea Diphenylthiourea 1,3-Diphenyl-2-thiourea DPTU Sulfocarbanilide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.732 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H12N2S | |
| Molar mass | 228.312 g/mol |
| Appearance | White powder |
| Density | 1.32 g/cm3 |
| Melting point | 154.5 °C (310.1 °F; 427.6 K) |
| Boiling point | decomposes |
| slightly soluble in water | |
| Solubility | very soluble in ethanol, diethyl ether, chloroform[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 164.7 °C (328.5 °F; 437.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Thiocarbanilide is commonly used as a vulcanization accelerator for rubber,[2] and as a stabilizer for PVC and PVDC. Its use as a vulcanization accelerator was discovered by BF Goodrich chemist George Oenslager.[3]
Reactions
Thiocarbanilide reacts with phosphorus pentachloride or hydrochloric acid, dilute sulfuric acid, acetic anhydride or iodine to produce phenyl isothiocyanate.
Toxicology
Oral, rat: LD50 = 50 mg/kg.
gollark: ```scheme(load-shared-object "/usr/lib/libc.so.6")(define fork (foreign-procedure #f "fork" () unsigned))(define waitpid (foreign-procedure #f "waitpid" (unsigned uptr unsigned) unsigned))(define mmap (foreign-procedure #f "mmap" (uptr unsigned unsigned unsigned unsigned unsigned) uptr))(define shmem (mmap 0 1024 3 0 0 0))(define pid (fork))(display "PID=")(display pid)(newline)(define apiomemetics (lambda (x y) (if (null? x) 0 (if (= (length x) 99) 1 (car x)))))(define prisond (lambda (x y) (if (= x y) (if (= x 1) '(-2 -2) '(-1 -1)) (if (= x 1) '(0 -3) '(-3 0)))))(if (= pid 0) ( (begin (display "hi from child") (foreign-set! 'integer-32 shmem 0 3) (newline) (exit) ) (begin (display "parent apioform") (newline) (waitpid pid 0 0) (display "hi from parent") (newline) (display (foreign-ref 'integer-32 shmem 0)) (newline) )))```Help?
gollark: "Invalid effective address"? What?
gollark: Well, most of them are in the code.
gollark: How exciting.
gollark: `grafting` instances can synchronize with each other then?
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–242, ISBN 0-8493-0594-2
- Hans-Wilhelm Engels, Herrmann-Josef Weidenhaupt, Manfred Pieroth, Werner Hofmann, Karl-Hans Menting, Thomas Mergenhagen, Ralf Schmoll, Stefan Uhrlandt "Rubber, 4. Chemicals and Additives" in Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_365.pub2
- Trumbull, H. L. (1933). "Accomplishments of the Medalist". Ind. Eng. Chem. 25 (2): 230–232. doi:10.1021/ie50278a030.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
