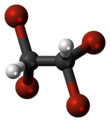
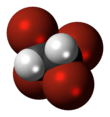
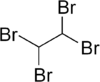
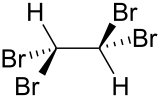
Tetrabromoethane
Tetrabromoethane (TBE) is a halogenated hydrocarbon, chemical formula C2H2Br4. Although three bromine atoms may bind to one of the carbon atoms creating 1,1,1,2-tetrabromoethane this is not thermodynamically favorable, so in practice tetrabromoethane is equal to 1,1,2,2-tetrabromoethane, where each carbon atom binds two bromine atoms.
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,2,2-Tetrabromoethane[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | TBE[2] | ||
| 1098321 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.083 | ||
| EC Number |
| ||
| MeSH | 1,1,2,2-tetrabromoethane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2504 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H2Br4 | |||
| Molar mass | 345.654 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 2.967 g mL−1 | ||
| Melting point | −1.0 °C; 30.3 °F; 272.2 K | ||
| Boiling point | 243.6 °C; 470.4 °F; 516.7 K | ||
| 630 mg L−1 (at 20 °C) | |||
| Vapor pressure | 10 Pa (at 20 °C) | ||
| -123.4·10−6 cm3/mol | |||
Refractive index (nD) |
1.637 | ||
| Thermochemistry | |||
Heat capacity (C) |
165.7 J K−1 mol−1 | ||
| Hazards | |||
| Safety data sheet | hells-confetti.com | ||
| GHS pictograms |  | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H319, H330, H412 | ||
| P260, P273, P284, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 97 °C (207 °F; 370 K) | ||
| 335 °C (635 °F; 608 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
|||
LC50 (median concentration) |
38 ppm (rat, 4 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1 ppm (14 mg/m3)[2] | ||
REL (Recommended) |
None established[2] | ||
IDLH (Immediate danger) |
8 ppm[2] | ||
| Related compounds | |||
Related alkanes |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
The compound has no commercial use. It has an unusually high density for an organic compound, near 3 g/mL, due largely to the four bromine atoms.[4] TBE is a liquid at room temperature, and is used to separate mineral ores from its supporting rock by means of preferential flotation. Quartz, feldspar, calcite, dolomite and other minerals with low density will float in TBE, while minerals such as sphalerite, galena and pyrite will sink. A related compound, bromoform, is also sometimes used in these applications, however, TBE is more practical because of its wider liquid range and lower vapor pressure.[4]
Safety
Permissible exposure limit is 1 ppm.[5] Cases of acute TBE poisoning are known as well.[6]
References
- "1,1,2,2-tetrabromoethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identifiction. Retrieved 20 June 2012.
- NIOSH Pocket Guide to Chemical Hazards. "#0009". National Institute for Occupational Safety and Health (NIOSH).
- "Acetylene tetrabromide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Organic based heavy liquids, heavyliquids.com
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- A B van Haaften (1969). "Acute Tetrabromoethane (Acetylene Tetrabromide) Intoxication in Man". American Industrial Hygiene Association. 30 (3): 251–256. doi:10.1080/00028896909343119. PMID 5793994.