Terthiophene
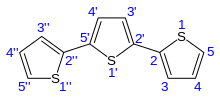
Terthiophene is the organic compound with the formula [C4H3S]2C4H2S. It is an oligomer of the heterocycle thiophene, a shorter oligomer is dithienyl, and the parent polymer is polythiophene. In the most common isomer of terthiophene, two thienyl groups are connected via their 2 positions to a central thiophene, also at the carbon atoms flanking the sulfur.
 | |
| Names | |
|---|---|
| IUPAC name
2,2':5',2"-terthiophene | |
| Other names
α-Terthienyl 2,5-Di(2-thienyl)thiophene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.168.218 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H8S3 | |
| Molar mass | 248.39 g/mol |
| Appearance | pale yellow solid |
| Melting point | 93-95 °C |
| insoluble | |
| Hazards | |
| Main hazards | flammable |
| S-phrases (outdated) | S22 S24/25 |
| Related compounds | |
Related compounds |
Thiophene polythiophene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation of terthiophene
Terthiophene is prepared by the nickel- or palladium-catalysed coupling reaction of 2,5-dibromothiophene with the Grignard reagent derived from 2-bromothiophene.[1]
Properties and applications
This isomer is a pigment in African marigolds (Tagetes spp.) and exhibits some biological activity because it sensitizes the formation of singlet oxygen.[2] It is responsible for the insecticidal activity of Tagetes minuta.[3]
Together with derivatives of 2,2'-bithiophene, various substituted terthiophenes occur naturally. Examples include 5,5''-dichloro-α-terthiophene, 5-chloro-α-terthiophene, 5-acetyl α-terthiophene, and 5-carboxyl bithiophene.[4]
Terthiophene has been employed as building block for the organic semi-conductor polythiophene.
See also
- Biphenyl
- Terphenyl
- Terpyridine
- Naphthalene, having fused rings.
References
- Smeets, B. J. J.; Meijer, R. H.; Meuldijk, J.; Vekemans, J. A. J. M. & Hulshof, L. A. (2003). "Process Design and Scale-Up of the Synthesis of 2,2':5',2"-Terthienyl". Organic Process Research & Development. 7 (1): 10–16. doi:10.1021/op020044n.
- Ciofalo, M.; Ponterini, G. (1994). "Generation of singlet oxygen by 2,2':5',2"-terthiophene and some of its derivatives". Journal of Photochemistry and Photobiology A. 83 (1): 1–6. doi:10.1016/1010-6030(94)03802-3. ISSN 1010-6030. CODEN: JPPCEJ.
- Perich, M. J.; Wells, C.; Bertsch, W.; Tredway, K. E. (1995). "Isolation of the insecticidal components of Tagetes minuta (Compositae) against mosquito larvae and adults". Journal of the American Mosquito Control Association. 11 (3): 307–310. PMID 8551298.
- Liu, Y.; Ye, M.; Guo, H. Z.; Zhao, Y. Y.; Guo, D. A. (2002). "New thiophenes from Echinops grijisii". Journal of Asian Natural Products Research. 4 (3): 175–178. doi:10.1080/1028602021000000071. ISSN 1028-6020. PMID 12118504. CODEN: JANRFI.