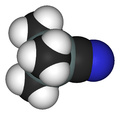
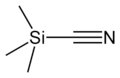Trimethylsilyl cyanide
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:[1]
- LiCN + (CH3)3SiCl → (CH3)3SiCN + LiCl
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
trimethylsilylformonitrile | |||
| Other names
Cyanotrimethylsilane; TMS cyanide; Trimethylsilylnitrile; Trimethylsilanecarbonitrile' Trimethylsilylcarbonitrile | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | TMSCN | ||
| ChemSpider | |||
| ECHA InfoCard | 100.028.780 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H9NSi | |||
| Molar mass | 99.208 g·mol−1 | ||
| Density | 0.793 g/mL at 20 °C | ||
| Melting point | 8 to 11 °C (46 to 52 °F; 281 to 284 K) | ||
| Boiling point | 114 to 117 °C (237 to 243 °F; 387 to 390 K) | ||
| hydrolyzes | |||
| Solubility | organic solvents | ||
Refractive index (nD) |
1.392 | ||
| Hazards | |||
| R-phrases (outdated) | R11 R26/27/28 R29 | ||
| S-phrases (outdated) | S16 S36/37/39 S45 | ||
| Flash point | 1 °C (34 °F; 274 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
The molecule exhibits the expected structure of a nitrile-like compound. The compound exists in a facile equilibrium with a small amount of the isomeric isocyanide (CH3)3SiNC.[2] By contrast, the nearly isostructural tert-butyl nitrile does not readily isomerize to tert-butyl isocyanide.
Reactions
Trimethylsilyl cyanide hydrolyzes to give hydrogen cyanide and trimethylsilanol:
- (CH3)3SiCN + H2O → (CH3)3SiOH + HCN
In its principal application, it adds across carbon-oxygen double bonds, for example in an aldehyde, to form a new carbon-carbon bond:
- RCH=O + (CH3)3SiC≡N → N≡C–CHR–OSi(CH3)3
The product is an O-silylated cyanohydrin.
One use of this reagent is to convert pyridine-N-oxides into 2-cyanopyridine. This transformation is best done in dichloromethane solution using dimethylcarbamoyl chloride as the activating electrophile. It is possible to use benzoyl chloride but the yields and regioselectivity of the addition of the cyano group are lower.
Safety
This chemical is rated as "Fatal if swallowed, in contact with skin or if inhaled" as it hydrolyzes in the presence of moisture to give hydrogen cyanide gas.
Disposal by using dilute solution of alkali hydroxide is recommended.[3]
References
- Livinghouse, T. (1990). "Trimethylsilyl Cyanide: Cyanosilation of p-Benzoquinone". Organic Syntheses.; Collective Volume, 7, p. 517
- Booth, M. R.; Frankiss, S. G. (1968). "Trimethylsilyl isocyanide". Chem. Commun. (21): 1347–1348. doi:10.1039/C19680001347.
- MSDS of trimethylsilyl cyanide. (PDF). Gelest. [Jun 13, 2019]