TIMM50
Mitochondrial import inner membrane translocase subunit TIM50 is an protein that in humans is encoded by the TIMM50 gene. Tim50 is a subunit of the Tim23 translocase complex in the inner mitochondrial membrane.[5][6] Mutations in TIMM50 can lead to epilepsy, severe intellectual disability, and 3-methylglutaconic aciduria.[7] TIMM50 expression is increased in breast cancer cells[8] and decreased in hypertrophic hearts.[9]
Structure
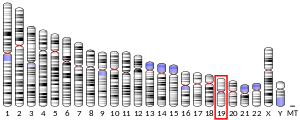
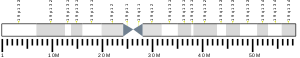
The TIMM50 gene is located on the q arm of chromosome 19 in position 13.2 and spans 13,373 base pairs.[6] The gene produces a 39.6 kDa protein composed of 353 amino acids.[10][11] This gene encodes a subunit of the TIM23 inner mitochondrial membrane translocase complex, which mediates the translocation of transit peptide-containing proteins across the mitochondrial inner membrane.[6]
Function
The Tim50 protein functions as the receptor subunit that recognizes the mitochondrial targeting signal, or presequence, on protein cargo that is destined for the mitochondrial inner membrane and matrix. This gene in human cells results in the release of cytochrome c and apoptosis.[6] This protein plays a role in maintaining the membrane permeability barrier. The intermembrane space domain of Tim50 induces the translocation pore of the TIM23 channel to close.[5][6]
Clinical significance
Missense mutations in TIMM50 often result in epilepsy or epileptic encephalopathy, severe intellectual disability, variable mitochondrial complex V deficiency, and 3-methylglutaconic aciduria, which is a key biomarker for mitochondrial membrane defects and mitochondrial dysfunction. Inheritance of TIMM50 is autosomal recessive.[7] Expression of the TIMM50 gene is increased in breast cancer cells. In such cells, overexpression of the Tim50 protein is linked to lack of cellular apoptosis and increased rates of proliferation.[8] Decreased TIMM50 expression in heart cells can lead to cardiac hypertrophy.[9]
Two patients, male and female siblings born to consanguineous Bedouin parents were presented, displaying involuntary abnormal movements, failure to thrive, hypsarrhythmia, bilateral optic atrophy, 3-methylglutaconic aciduria, and slightly elevated plasma lactate levels. Both began walking independently at only 3 years and initially received favorably ACTH therapy until switching to a treatment of Valproate with either Sabril or Topamax, which resulted in seizures completely disappearing. Two more patients, male and female siblings born to first-cousin parents of Muslim origin were also presented, displaying myoclonic and tonic seizures, abnormal EEG, brain atrophy, delayed psychomotor development and 3-methylglutaconic aciduria. Treatment of Lamictal combined with Valproate was effective in controlling the seizures.[7]
Interactions
Within the TIM23 complex, the Tim50 subunit directly interacts with TIMM23. The TIM23 complex interacts with the TIMM44 component of the PAM complex and with DNAJC15. An isoform of Tim50 interacts with COIL and snRNPs.[12]
References
- GRCh38: Ensembl release 89: ENSG00000105197 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000003438 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Yamamoto H, Esaki M, Kanamori T, Tamura Y, Nishikawa S, Endo T (November 2002). "TIM50 is a subunit of the Tim23 complex which links protein translocation across the outer and inner mitochondrial membranes". Cell. 111 (4): 519–28. doi:10.1016/S0092-8674(02)01053-X. PMID 12437925.
- "Entrez Gene: TIMM50 translocase of inner mitochondrial membrane 50 homolog (S. cerevisiae)".
- Shahrour MA, Staretz-Chacham O, Dayan D, Stephen J, Weech A, Damseh N, et al. (May 2017). "Mitochondrial epileptic encephalopathy, 3-methylglutaconic aciduria and variable complex V deficiency associated with TIMM50 mutations". Clinical Genetics. 91 (5): 690–696. doi:10.1111/cge.12855. PMID 27573165.
- Gao SP, Sun HF, Jiang HL, Li LD, Hu X, Xu XE, Jin W (January 2016). "Loss of TIM50 suppresses proliferation and induces apoptosis in breast cancer". Tumor Biology. 37 (1): 1279–87. doi:10.1007/s13277-015-3878-0. PMID 26289846.
- Tang K, Zhao Y, Li H, Zhu M, Li W, Liu W, et al. (April 2017). "Translocase of Inner Membrane 50 Functions as a Novel Protective Regulator of Pathological Cardiac Hypertrophy". Journal of the American Heart Association. 6 (4): e004346. doi:10.1161/JAHA.116.004346. PMC 5532988. PMID 28432072.
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, et al. (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- "Mitochondrial import inner membrane translocase subunit TIM50". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- "TIMM50 - Mitochondrial import inner membrane translocase subunit TIM50 precursor - Homo sapiens (Human) - TIMM50 gene & protein". www.uniprot.org. Retrieved 2018-07-25.
Further reading
- Xu H, Somers ZB, Robinson ML, Hebert MD (July 2005). "Tim50a, a nuclear isoform of the mitochondrial Tim50, interacts with proteins involved in snRNP biogenesis". BMC Cell Biology. 6 (1): 29. doi:10.1186/1471-2121-6-29. PMC 1177934. PMID 16008839.
- Guo Y, Cheong N, Zhang Z, De Rose R, Deng Y, Farber SA, Fernandes-Alnemri T, Alnemri ES (June 2004). "Tim50, a component of the mitochondrial translocator, regulates mitochondrial integrity and cell death". The Journal of Biological Chemistry. 279 (23): 24813–25. doi:10.1074/jbc.M402049200. PMID 15044455.
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, et al. (February 2004). "A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway". Nature Cell Biology. 6 (2): 97–105. doi:10.1038/ncb1086. PMID 14743216.
- Yuryev A, Wennogle LP (February 2003). "Novel raf kinase protein-protein interactions found by an exhaustive yeast two-hybrid analysis". Genomics. 81 (2): 112–25. doi:10.1016/S0888-7543(02)00008-3. PMID 12620389.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.