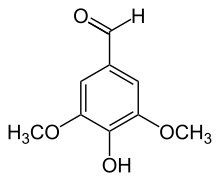
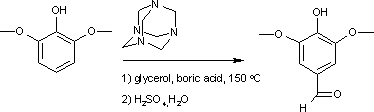Syringaldehyde
Syringaldehyde is an organic compound that occurs in trace amounts widely in nature. Some species of insects use syringaldehyde in their chemical communication systems. Scolytus multistriatus uses it as a signal to find a host tree during oviposition.[1]
 | |
| Names | |
|---|---|
| IUPAC name
4-Hydroxy-3,5-dimethoxybenzaldehyde | |
| Other names
3,5-Dimethoxy-4-hydroxybenzene carbonal, Gallaldehyde 3,5-dimethyl ether, 4-Hydroxy-3,5-dimethoxybenzaldehyde, Syringic aldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.698 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O4 | |
| Molar mass | 182.17 g/mol |
| Appearance | colorless solid |
| Density | 1.01 g/cm3 |
| Melting point | 110 to 113 °C (230 to 235 °F; 383 to 386 K) |
| Boiling point | 192 to 193 °C (378 to 379 °F; 465 to 466 K) at 19 kPa |
| Insoluble | |
| Hazards | |
| Main hazards | Irritant (Xi) |
| Safety data sheet | External MSDS |
| S-phrases (outdated) | S24/25, S28A, S37, S45 |
| NFPA 704 (fire diamond) | |
| Flash point | > 110 °C (230 °F; 383 K) c.c. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because it contains many functional groups, it can be classified in many ways - aromatic, aldehyde, phenol. It is a colorless solid (impure samples appear yellowish) that is soluble in alcohol and polar organic solvents. Its refractive index is 1.53.
Natural sources
Syringaldehyde can be found naturally in the wood of spruce and maple trees.[2]
Syringaldehyde is also formed in oak barrels and extracted into whisky, which it gives spicy, smoky, hot and smoldering wood aromas.
Preparation
This compound may be prepared from syringol by the Duff reaction:[3]
See also
References
- Vanillin and Syringaldehyde as Attractants for Scolytus multistriatus (Coleoptera: Scolytidae). Meyer H.J. and Norris D.M., Annals of the Entomological Society of America, 17 July 1967, Volume 60, Number 4, pages 858-859, (abstract)
- R.H.J. Creighton; J.L. McCarthy; H. Hibbert (1941). "Aromatic Aldehyde from Spruce and Maple Woods". J. Am. Chem. Soc. 63: 312. doi:10.1021/ja01846a501.
- C. F. H. Allen and Gerhard W. Leubner (1963). "Syringic aldehyde". Organic Syntheses.; Collective Volume, 4, p. 866