Superbase
A superbase is a compound that has a high affinity for protons. Superbases are valuable in organic chemistry, which abounds in very weak acids. Reactions involving superbases often require special techniques since they're easily destroyed by water, atmospheric carbon dioxide, and, often, oxygen. A common superbase is lithium diisopropylamide.
Definitions
A "superbase" has been defined as an organic compound whose basicity is greater than that of proton sponge, which has a conjugate pKa of 12.1.[1] Strong superbases can be prepared from extending the hydrogen bond network of multiple amino groups substituted on an aromatic core.[2] Superbases are of great interest to practicing organic chemists due to their reactivity as well as good solubility in organic solvents.
IUPAC broadly defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide."[3] Caubère defines superbases as "bases resulting from a mixing of two (or more) bases leading to new basic species possessing inherent new properties. The term superbase does not mean a base is thermodynamically and/or kinetically stronger than another, instead it means that a basic reagent is created by combining the characteristics of several different bases."[4]
Superbases have also been defined semi-quantitatively as any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than Alder's canonical proton sponge (1,8-bis-(dimethylamino)-naphthalene).[5]
Superbases have been described and used since the 1850s.[6]
Organic superbases

Organic superbases are almost always charge-neutral, amine-containing species. Despite enormous proton affinity, some organosuperbases exhibit low nucleophilicity. Increasingly important in organic synthesis, these include the phosphazenes, amidines, and guanidines. Other organic compounds also meet the physicochemical or structural definitions of 'superbase'. Proton chelators like the aromatic proton sponges and the bispidines are also superbases. Multicyclic polyamines, like DABCO might also be loosely included in this category.[8]
Organometallic
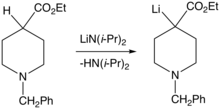
Organometallic compounds of electropositive metals are superbases, but they are generally strong nucleophiles. Examples include organolithium and organomagnesium (Grignard reagent) compounds. Another type of organometallic superbase has a reactive metal exchanged for a hydrogen on a heteroatom, such as oxygen (unstabilized alkoxides) or nitrogen (metal amides such as lithium diisopropylamide).
The Schlosser base (or Lochmann-Schlosser base), the combination of n-butyllithium and potassium tert-butoxide, is commonly cited as a superbase. n-Butyllithium and potassium tert-butoxide form a mixed aggregate of greater reactivity than either component reagent.[10]
Inorganic
Inorganic superbases are typically salt-like compounds with small, highly charged anions, e.g. lithium hydride, potassium hydride, and sodium hydride. Such species are insoluble, but the surfaces of these materials are highly reactive and slurries are useful in synthesis.
Superbases in organic chemistry
Superbases are used in organocatalysis.[11]
The strongest superbases
The list of the strongest superbases as ranked by their calculated gas phase basicity (proton affinity) is given below.
- Ortho-diethynylbenzene dianion
- Meta-diethynylbenzene dianion
- Para-diethynylbenzene dianion
- Lithium monoxide anion
- Methyl anion
See also
References
- Pozharskii, Alexander F.; Ozeryanskii, Valery A. (2012). "Proton Sponges and Hydrogen Transfer Phenomena". Mendeleev Communications. 22 (3): 117–124. doi:10.1016/j.mencom.2012.05.001.
- Bachrach, Steven M. (2012-11-02). "Extended Hydrogen Bond Network Enabled Superbases". Organic Letters. 14 (21): 5598–5601. doi:10.1021/ol302722s. ISSN 1523-7060. PMID 23072544.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "superacid". doi:10.1351/goldbook.S06135
- Caubère, P (1993). "Unimetal super bases". Chemical Reviews. 93 (6): 2317–2334. doi:10.1021/cr00022a012.
- Raczynska, Ewa D.; Decouzon, Michele; Gal, Jean-Francois; Maria, Pierre-Charles; Wozniak, Krzysztof; Kurg, Rhio; Carins, Stuart N. (3 June 2010). "ChemInform Abstract: Superbases and Superacids in the Gas Phase". ChemInform. 31 (33): no. doi:10.1002/chin.200033267.
- "BBC - h2g2 - History of Chemistry - Acids and Bases". Retrieved 2009-08-30.
- Verkade, John G.; Urgaonkar, Sameer; Verkade, John G.; Urgaonkar, Sameer (2012). "Proazaphosphatrane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00702.pub2.
- Superbases for Organic Synthesis Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009.
- Jianshe Kong, Tao Meng, Pauline Ting, and Jesse Wong (2010). "Preparation of Ethyl 1-Benzyl-4-Fluoropiperidine-4-Carboxylate". Organic Syntheses. 87: 137. doi:10.15227/orgsyn.087.0137.CS1 maint: multiple names: authors list (link)
- Schlosser, M. (1988). "Superbases for organic synthesis". Pure Appl. Chem. 60 (11): 1627–1634. doi:10.1351/pac198860111627.
- MacMillan, David W. C. (2008). "The advent and development of organocatalysis". Nature. 455 (7211): 304–308. Bibcode:2008Natur.455..304M. doi:10.1038/nature07367.
