Sortilin 1
Sortilin (SORT1) is a protein that in humans is encoded by the SORT1 gene on chromosome 1.[5] This protein is a type I membrane glycoprotein in the vacuolar protein sorting 10 protein (Vps10p) family of sorting receptors. While it is ubiquitously expressed in many tissues,[6] sortilin is most abundant in the central nervous system.[7] At the cellular level, sortilin functions in protein transport between the Golgi apparatus, endosome, lysosome, and plasma membrane, leading to its involvement in multiple biological processes such as glucose and lipid metabolism as well as neural development and cell death.[8][9][10][11][12] Moreover, the function and role of sortilin is now emerging in several major human diseases such as atherosclerosis and coronary artery disease, Alzheimer’s disease, and cancer.[13][14][15] The SORT1 gene also contains one of 27 loci associated with increased risk of coronary artery disease.[16]
Structure
Gene
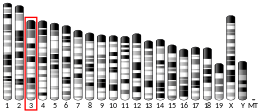
The SORT1 gene resides on chromosome 1 at the band 1p13.3 and includes 23 exons.[5] This gene encodes 2 isoforms through alternative splicing.[17]
Protein
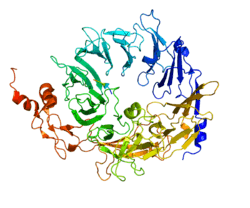
Sortilin is a member of the Vps10p sorting receptor family.[7] Crystallization studies of the protein reveal that, when complexed with the ligand neurotensin, the Vps10 ectodomain of sortilin forms a ten-bladed beta-propeller structure with an inner tunnel that contains multiple ligand binding sites.[18] To prevent premature ligand binding during its synthesis, the precursor protein of sortilin contains a 44-amino acid pro-peptide that serves as a chaperone for the Vps10p domain.[19] In addition, two hydrophobic loops have been detected in this domain and act to anchor the protein in the cell membrane.[20] Sortilin has also been shown to undergo a conformational change and form a protein dimer in acidic conditions similar to ones found in the endosome, indicating a double mechanism for low pH-induced ligand release and possibly signaling towards recycling of the receptor. [21]
Function
In humans, sortilin is expressed over a wide range of cell types and tissues such as the brain, spinal cord, adrenal gland, thyroid, B-lymphocytes, adipocytes, skeletal muscle, and heart.[22] As a sorting receptor on the cell surface and on the endoplasmic reticulum-Golgi apparatus within the cell, sortilin is involved in the transport of a wide variety of intracellular proteins between the trans-Golgi network, endosome, lysosome, and secretory granules, as well as the plasma membrane.[8] This molecular function enables sortilin to participate in various biological processes, including the transport of GLUT4 to the plasma membrane of fat and skeletal muscle cells in response to insulin.[9] It also mediates the interaction between proNGF and the p75NTR:sortilin complex by acting as a co-receptor to signal cell death.[12][19] The fine regulation of the brain-derived neurotrophic factor (BDNF) by sortilin is required for both neuronal and tumor cell survival.[23] Moreover, sortilin has been implicated in LDL-cholesterol metabolism, VLDL secretion, and PCSK9 secretion, and thus plays a role in the development of atherosclerotic lesions.[10][11] Other processes involving sortilin include endocytosis,[8] negative regulation of lipoprotein lipase activity,[24] myotube differentiation,[25] ossification,[26] and regulation of gene expression.[25]
Clinical significance
Given its function in facilitating lysosomal degradation or recycling of ligands in lipid metabolism[11][13][27][28][29] and the neural system,[30] sortilin likely plays an important role in the underlying mechanisms and pathophysiology of atherogenesis and coronary artery disease, as well as in neurological disorders. For example, sortilin has been identified as an important receptor for brain apolipoprotein E (APOE) metabolism, which is implicated in the underlying mechanisms of Alzheimer’s disease.[30][31][32][33] A significant role for sortilin has recently also been reported in the field of oncology, as it has been detected in several cancer cell lines. Notably, human cancerous epithelial cells exhibited increased levels of sortilin as compared to normal epithelial tissues. Furthermore, it appears that sortilin participates in the progression of breast cancer and contributes to tumor cell adhesion and invasion.[14][15]
Clinical marker
In 2007, chromosome 1p13.3 was identified as a promising locus through a genome-wide approach in patients with coronary artery disease.[34] Subsequently, accumulating evidence suggests that the SORT1 gene at the 1p13 locus is an important risk factor for coronary artery disease, which is attributed to lipid metabolism disorders.[34][35][36] As the role of sortilin in lipid metabolism and the development of atherosclerosis has been established, a recent study further reported that increased release of soluble sortilin from platelets, measured as circulating sortilin, may be associated with in vivo platelet activation.[37] This observation also indicates that sortilin has a potential application as a clinical biomarker for diagnosis and prognosis.[10][37] Additionally, a multi-locus genetic risk score study, based on a combination of 27 loci including the SORT1 gene, identified individuals at increased risk for both incident and recurrent coronary artery disease events, as well as an enhanced clinical benefit from statin therapy. The study was based on a community cohort study (the Malmo Diet and Cancer study) and four additional randomized controlled trials of primary prevention cohorts (JUPITER and ASCOT) and secondary prevention cohorts (CARE and PROVE IT-TIMI 22).[16]
Interactions
Sortilin has been shown to interact with GGA1[38] and GGA2.[8][38]
Interactive Pathway Map
Sortilin participates in interactions within the trans-Golgi network vesicle budding and BDNF signaling pathways.
See also
References
- GRCh38: Ensembl release 89: ENSG00000134243 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000068747 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: SORT1 sortilin 1".
- "BioGPS - your Gene Portal System". biogps.org. Retrieved 2016-08-16.
- Andersen JL, Schrøder TJ, Christensen S, Strandbygård D, Pallesen LT, García-Alai MM, Lindberg S, Langgård M, Eskildsen JC, David L, Tagmose L, Simonsen KB, Maltas PJ, Rønn LC, de Jong IE, Malik IJ, Egebjerg J, Karlsson JJ, Uppalanchi S, Sakumudi DR, Eradi P, Watson SP, Thirup S (February 2014). "Identification of the first small-molecule ligand of the neuronal receptor sortilin and structure determination of the receptor-ligand complex". Acta Crystallographica Section D. 70 (Pt 2): 451–60. doi:10.1107/S1399004713030149. PMC 3940197. PMID 24531479.
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM (May 2001). "The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein". The EMBO Journal. 20 (9): 2180–90. doi:10.1093/emboj/20.9.2180. PMC 125444. PMID 11331584.
- Huang G, Buckler-Pena D, Nauta T, Singh M, Asmar A, Shi J, Kim JY, Kandror KV (October 2013). "Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin". Molecular Biology of the Cell. 24 (19): 3115–22. doi:10.1091/mbc.E12-10-0765. PMC 3784384. PMID 23966466.
- Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ (February 2015). "Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis". Circulation Research. 116 (5): 789–96. doi:10.1161/CIRCRESAHA.116.305811. PMC 4602371. PMID 25593281.
- Kjolby M, Nielsen MS, Petersen CM (April 2015). "Sortilin, encoded by the cardiovascular risk gene SORT1, and its suggested functions in cardiovascular disease". Current Atherosclerosis Reports. 17 (4): 496. doi:10.1007/s11883-015-0496-7. PMID 25702058.
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM (February 2004). "Sortilin is essential for proNGF-induced neuronal cell death". Nature. 427 (6977): 843–8. doi:10.1038/nature02319. PMID 14985763.
- Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E (April 2016). "Sortilin mediates vascular calcification via its recruitment into extracellular vesicles". The Journal of Clinical Investigation. 126 (4): 1323–36. doi:10.1172/JCI80851. PMC 4811143. PMID 26950419.
- Roselli S, Pundavela J, Demont Y, Faulkner S, Keene S, Attia J, Jiang CC, Zhang XD, Walker MM, Hondermarck H (April 2015). "Sortilin is associated with breast cancer aggressiveness and contributes to tumor cell adhesion and invasion". Oncotarget. 6 (12): 10473–86. doi:10.18632/oncotarget.3401. PMC 4496368. PMID 25871389.
- Wilson CM, Naves T, Al Akhrass H, Vincent F, Melloni B, Bonnaud F, Lalloué F, Jauberteau MO (2016-02-01). "A new role under sortilin's belt in cancer". Communicative & Integrative Biology. 9 (1): e1130192. doi:10.1080/19420889.2015.1130192. PMC 4802778. PMID 27066187.
- Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield MJ, Devlin JJ, Nordio F, Hyde CL, Cannon CP, Sacks FM, Poulter NR, Sever PS, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS (June 2015). "Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials". Lancet. 385 (9984): 2264–71. doi:10.1016/S0140-6736(14)61730-X. PMC 4608367. PMID 25748612.
- "SORT1 - Sortilin precursor - Homo sapiens (Human) - SORT1 gene & protein". www.uniprot.org. Retrieved 2016-08-16.
- Quistgaard EM, Madsen P, Grøftehauge MK, Nissen P, Petersen CM, Thirup SS (January 2009). "Ligands bind to Sortilin in the tunnel of a ten-bladed beta-propeller domain". Nature Structural & Molecular Biology. 16 (1): 96–8. doi:10.1038/nsmb.1543. PMID 19122660.
- Nykjaer A, Willnow TE (April 2012). "Sortilin: a receptor to regulate neuronal viability and function". Trends in Neurosciences. 35 (4): 261–70. doi:10.1016/j.tins.2012.01.003. PMID 22341525.
- Quistgaard EM, Grøftehauge MK, Madsen P, Pallesen LT, Christensen B, Sørensen ES, Nissen P, Petersen CM, Thirup SS (September 2014). "Revisiting the structure of the Vps10 domain of human sortilin and its interaction with neurotensin". Protein Science. 23 (9): 1291–300. doi:10.1002/pro.2512. PMC 4243999. PMID 24985322.
- Leloup N, Loessl P, Meijer DH, Brennich M, Heck AJ, Thies-Weesie DM, Janssen BJ (November 2017). "Low pH-induced conformational change and dimerization of sortilin triggers endocytosed ligand release". Nature Communications. 8 (1708). doi:10.1038/s41467-017-01485-5. PMC 5700061. PMID 29167428.
- Schmidt V, Willnow TE (February 2016). "Protein sorting gone wrong--VPS10P domain receptors in cardiovascular and metabolic diseases" (PDF). Atherosclerosis. 245: 194–9. doi:10.1016/j.atherosclerosis.2015.11.027. PMID 26724530.
- Akil H, Perraud A, Mélin C, Jauberteau MO, Mathonnet M (2011-01-01). "Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival". PLOS ONE. 6 (9): e25097. doi:10.1371/journal.pone.0025097. PMC 3180371. PMID 21966426.
- Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM (March 1999). "Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase". The Journal of Biological Chemistry. 274 (13): 8832–6. doi:10.1074/jbc.274.13.8832. PMID 10085125.
- Ariga M, Nedachi T, Katagiri H, Kanzaki M (April 2008). "Functional role of sortilin in myogenesis and development of insulin-responsive glucose transport system in C2C12 myocytes". The Journal of Biological Chemistry. 283 (15): 10208–20. doi:10.1074/jbc.M710604200. PMID 18258592.
- Maeda S, Nobukuni T, Shimo-Onoda K, Hayashi K, Yone K, Komiya S, Inoue I (October 2002). "Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization". Journal of Cellular Physiology. 193 (1): 73–9. doi:10.1002/jcp.10151. PMID 12209882.
- Strong A, Rader DJ (June 2012). "Sortilin as a regulator of lipoprotein metabolism". Current Atherosclerosis Reports. 14 (3): 211–8. doi:10.1007/s11883-012-0248-x. PMC 7089359. PMID 22538429.
- Zhong LY, Cayabyab FS, Tang CK, Zheng XL, Peng TH, Lv YC (September 2016). "Sortilin: A novel regulator in lipid metabolism and atherogenesis". Clinica Chimica Acta. 460: 11–7. doi:10.1016/j.cca.2016.06.013. PMID 27312323.
- Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A (September 2010). "Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export". Cell Metabolism. 12 (3): 213–23. doi:10.1016/j.cmet.2010.08.006. PMID 20816088.
- Carlo AS (2013-10-01). "Sortilin, a novel APOE receptor implicated in Alzheimer disease". Prion. 7 (5): 378–82. doi:10.4161/pri.26746. PMC 4134342. PMID 24121631.
- Jin C, Liu X, Zhang F, Wu Y, Yuan J, Zhu J, Zhang F, Wang G, Cheng Z (2013-01-01). "An updated meta-analysis of the association between SORL1 variants and the risk for sporadic Alzheimer's disease". Journal of Alzheimer's Disease. 37 (2): 429–37. doi:10.3233/JAD-130533. PMID 23948893.
- Piscopo P, Tosto G, Belli C, Talarico G, Galimberti D, Gasparini M, Canevelli M, Poleggi A, Crestini A, Albani D, Forloni G, Lucca U, Quadri P, Tettamanti M, Fenoglio C, Scarpini E, Bruno G, Vanacore N, Confaloni A (2015-01-01). "SORL1 Gene is Associated with the Conversion from Mild Cognitive Impairment to Alzheimer's Disease". Journal of Alzheimer's Disease. 46 (3): 771–6. doi:10.3233/JAD-141551. PMID 25881907.
- Andersson CH, Hansson O, Minthon L, Andreasen N, Blennow K, Zetterberg H, Skoog I, Wallin A, Nilsson S, Kettunen P (July 2016). "A Genetic Variant of the Sortilin 1 Gene is Associated with Reduced Risk of Alzheimer's Disease". Journal of Alzheimer's Disease. 53: 1353–63. doi:10.3233/JAD-160319. PMC 5147507. PMID 27392867.
- Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, König IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H (August 2007). "Genomewide association analysis of coronary artery disease". The New England Journal of Medicine. 357 (5): 443–53. doi:10.1056/NEJMoa072366. PMC 2719290. PMID 17634449.
- Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. (October 2015). "A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease". Nature Genetics. 47 (10): 1121–30. doi:10.1038/ng.3396. PMC 4589895. PMID 26343387.
- Zeller T, Blankenberg S, Diemert P (January 2012). "Genomewide association studies in cardiovascular disease--an update 2011". Clinical Chemistry. 58 (1): 92–103. doi:10.1373/clinchem.2011.170431. PMID 22125304.
- Ogawa K, Ueno T, Iwasaki T, Kujiraoka T, Ishihara M, Kunimoto S, Takayama T, Kanai T, Hirayama A, Hattori H (June 2016). "Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors". Atherosclerosis. 249: 110–5. doi:10.1016/j.atherosclerosis.2016.03.041. PMID 27085161.
- Jacobsen L, Madsen P, Nielsen MS, Geraerts WP, Gliemann J, Smit AB, Petersen CM (January 2002). "The sorLA cytoplasmic domain interacts with GGA1 and -2 and defines minimum requirements for GGA binding". FEBS Letters. 511 (1–3): 155–8. doi:10.1016/S0014-5793(01)03299-9. PMID 11821067.
Further reading
- Vincent JP, Mazella J, Kitabgi P (July 1999). "Neurotensin and neurotensin receptors". Trends in Pharmacological Sciences. 20 (7): 302–9. doi:10.1016/S0165-6147(99)01357-7. PMID 10390649.
- Mazella J (January 2001). "Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking?". Cellular Signalling. 13 (1): 1–6. doi:10.1016/S0898-6568(00)00130-3. PMID 11257441.
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK (February 1997). "Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography". The Journal of Biological Chemistry. 272 (6): 3599–605. doi:10.1074/jbc.272.6.3599. PMID 9013611.
- Lin BZ, Pilch PF, Kandror KV (September 1997). "Sortilin is a major protein component of Glut4-containing vesicles". The Journal of Biological Chemistry. 272 (39): 24145–7. doi:10.1074/jbc.272.39.24145. PMID 9305862.
- Tauris J, Ellgaard L, Jacobsen C, Nielsen MS, Madsen P, Thøgersen HC, Gliemann J, Petersen CM, Moestrup SK (June 1998). "The carboxy-terminal domain of the receptor-associated protein binds to the Vps10p domain of sortilin". FEBS Letters. 429 (1): 27–30. doi:10.1016/S0014-5793(98)00559-6. PMID 9657377.
- Mazella J, Zsürger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP (October 1998). "The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor". The Journal of Biological Chemistry. 273 (41): 26273–6. doi:10.1074/jbc.273.41.26273. PMID 9756851.
- Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P (February 1999). "Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding". The EMBO Journal. 18 (3): 595–604. doi:10.1093/emboj/18.3.595. PMC 1171152. PMID 9927419.
- Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM (March 1999). "Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase". The Journal of Biological Chemistry. 274 (13): 8832–6. doi:10.1074/jbc.274.13.8832. PMID 10085125.
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM (May 2001). "The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein". The EMBO Journal. 20 (9): 2180–90. doi:10.1093/emboj/20.9.2180. PMC 125444. PMID 11331584.
- Takatsu H, Katoh Y, Shiba Y, Nakayama K (July 2001). "Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains". The Journal of Biological Chemistry. 276 (30): 28541–5. doi:10.1074/jbc.C100218200. PMID 11390366.
- Hampe W, Rezgaoui M, Hermans-Borgmeyer I, Schaller HC (June 2001). "The genes for the human VPS10 domain-containing receptors are large and contain many small exons". Human Genetics. 108 (6): 529–36. doi:10.1007/s004390100504. PMID 11499680.
- Shiba T, Takatsu H, Nogi T, Matsugaki N, Kawasaki M, Igarashi N, Suzuki M, Kato R, Earnest T, Nakayama K, Wakatsuki S (February 2002). "Structural basis for recognition of acidic-cluster dileucine sequence by GGA1". Nature. 415 (6874): 937–41. doi:10.1038/415937a. PMID 11859376.
- Maeda S, Nobukuni T, Shimo-Onoda K, Hayashi K, Yone K, Komiya S, Inoue I (October 2002). "Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization". Journal of Cellular Physiology. 193 (1): 73–9. doi:10.1002/jcp.10151. PMID 12209882.
- Martin S, Navarro V, Vincent JP, Mazella J (October 2002). "Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line". Gastroenterology. 123 (4): 1135–43. doi:10.1053/gast.2002.36000. PMID 12360476.
- Navarro V, Vincent JP, Mazella J (November 2002). "Shedding of the luminal domain of the neurotensin receptor-3/sortilin in the HT29 cell line". Biochemical and Biophysical Research Communications. 298 (5): 760–4. doi:10.1016/S0006-291X(02)02564-0. PMID 12419319.
- Martin S, Vincent JP, Mazella J (February 2003). "Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia". The Journal of Neuroscience. 23 (4): 1198–205. doi:10.1523/JNEUROSCI.23-04-01198.2003. PMID 12598608.
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR (December 2003). "The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin". The EMBO Journal. 22 (24): 6430–7. doi:10.1093/emboj/cdg629. PMC 291824. PMID 14657016.