Solid-state electrolyte
A solid-state electrolyte (SSE) is a solid ionic conductor electrolyte and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery.[1][2] The main advantages are the increased safety, no issues of leakages of toxic organic liquids, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability.[3] This makes it possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite (372 mAh g−1) is the first step in the realization of a lighter, thinner and cheaper rechargeable battery.[4] Moreover this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle.[5] Despite the promising advantages there are still some limitations that are hindering the transition of SSEs from academic research to large-scale production, however many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.[6][7]

History
The first inorganic solid-state electrolytes were discovered by M. Faraday in the nineteenth century, the silver sulfide (Ag2S) and lead(II) fluoride (PbF2).[8] The first polymeric material able to conduct ions at the solid-state was PEO, discovered in the 1970s by V. Wrigh. The importance of the discovery was recognized in the early of 1980s.[9][10]
However, unresolved fundamental issues remain in order to fully understand the behavior of all-solid batteries, especially in the area of electrochemical interfaces.[11] In recent years the need of safety and performances improvements with respect to the state-of-the-art Li-ion chemistry are making solid-state batteries very appealable and are now considered an encouraging technology to satisfy the need for long range battery electric vehicles of the near future.
In March 2020, the Samsung Advanced Institute of Technology (SAIT) published the research on an all-solid-state battery (ASSB) using an argyrodite-based solid-state electrolyte with a demonstrated energy density of 900 Wh L−1 and a stable cyclability of more than 1000 cycles, reaching for the first time a value close to the 1000 Wh L−1.[12]
Properties
In order to design a SSE with the optimal performances, several properties have to be met:[13]
- high ionic conductivity (at least higher than 10−4 S cm−1) that can be measured through electrochemical impedance spectroscopy (EIS) analysis[14]
- high ionic transference number (the closest possible to 1) that can be measure through a combination of chronoamperometry (CA) and EIS analysis[15]
- wide electrochemical stability windows (ESW) (at least 4-5 V) that can be measured through linear sweep voltammetry (LSV) or cyclic voltammetry (CV)[16]
- high mechanical strength (at least tens of MPa) that can be measured through a traditional tensile test
- high compatibility with the electrode material that can be measured through EIS analysis repeated over more consecutive days.[17]
Categories
SSEs have the same role of a traditional liquid electrolyte and they are classified into all-solid-state electrolyte and quasi-solid-state electrolyte (QSSE). All-solid-state electrolytes are furthermore divided into inorganic solid electrolyte (ISE), solid polymer electrolyte (SPE) and composite polymer electrolyte (CPE). On the other hand, a QSSE, also called gel polymer electrolyte (GPE), is a freestanding membrane that contains a certain amount of liquid compontent immobilized inside the solid matrix. In general the nomenclatures SPE and GPE are used interchangeably but they have a substantially different ionic conduction mechanism: SPEs conducts ions through the interaction with the susbtitutional groups of the polymer chains, while GPEs conducts ions mainly in the solvent or plasticizer.[18]
All-solid-state electrolyte
All-solid-state electrolyte are divided into inorganic solid electrolyte (ISE), solid polymer electrolite (SPE) and composite polymer electrolyte (CPE). They are solid at room temperature and the ionic movement occurs at the solid-state. Their main advantage is the complete removal of any liquid component aimed to a greatly enhanced safety of the overall device. The main limitation is the ionic conductivity that tends to be much lower compared to a liquid counterpart.[19]
- Inorganic solid electrolyte (ISE)
Inorganic solid electrolyte (ISE) are a particular type of all-solid-state electrolyte that is constituted by an inorganic material in the crystalline or glassy state, that conducts ion by diffusion through the lattice.[20] The main advantages of this class of solid-state electrolyte are the high ionic conductivity (of the order of few mS cm−2 at room-temperature), high modulus (of the order of GPa) and high transfer number compared to other classes of SSEs.[21] They are generally brittle and with this comes a low compatibility and stability towards the electrode, with a rapidly increasing interfacial resistance and a complicated scale-up from academic to industry.[22] They can be oxides, sulfides or phosphates-based and the crystalline structures include LISICON (lithium superionic conductor) (e.g. LGPS, LiSiPS, LiPS), argyrodite-like (e.g. Li6PS5X, X = Cl, Br, I),[23] garnets (LLZO),[24] NASICON (sodium superionic conductor) (e.g. LTP, LATP, LAGP),[25] lithium nitrides (e.g. Li3N),[26] lithium hydrides (LiBH4),[27] perovskites (e.g. LLTO),[28] lithium halides (LYC, LYB).[29] Some ISEs can be glass ceramics assuming an amorfous state instead of a regular crystalline structure, popular examples are lithium phosporus oxynitride (LIPON)[30] and the lithium thiophosphates (Li2S–P2S5).[31]
- Solid polymer electrolyte (SPE)
Solid polymer electrolyte (SPE) are defined as a solvent-free salt solution in a polymer host material that conducts ions through the polymer chains. Compared to ISEs, SPEs are much easier to process, generally by solution casting, making them greatly compatibile with large-scale manufacturing processes. Moreover, they possess higher elasticity and plasticity giving stability at the interface, flexibility and improved resistance to volume changes during operation.[32] A good dissolution of Li salts, low glass transition temperature (Tg), electrochemical compatibility with most common electrode materials, a low degree of crystallinity, mechanical stability, low temperature sensitivity are all characteristics for the ideal SPE candidate.[33] In general though the ionic conductivity is lower than the ISEs and their rate capability is restricted, limiting fast charging.[34] PEO-based SPE is the first solid-state polymer in which ionic conductivity was demonstrated both through inter and intra molecular through ion hopping, thanks to the segmental motion of the polymeric chains[35] because of the great ion complexation capability of the ether groups, but they suffer from the low room-temperature ionic conductivity (10−5 S cm−1)[36] due to the high degree of cristallinity. The main alternatives to polyether-based SPEs are polycarbonates,[37] polyesters,[38] polynitriles (e.g. PAN),[39] polyalcohols (e.g. PVA),[40] polyamines (e.g. PEI),[41] polysiloxane (e.g. PDMS)[42][43] and fluoropolymers (e.g. PVDF, PVDF-HFP).[44] Bio-polymers like lignin,[45] chitosan[46] and cellulose[47] are also gaining a lot of interest as standalone SPEs or blended with other polymers, on one side for their environmentally friendliness and on the other for their high complexation capability on the salts. Furthermore different strategies are considered to increase the ionic conductivity of SPEs and the amorfous-to-crystalline ratio.[48]
With the introduction of particles as fillers inside the polymer solution, a composite polymer electrolyte (CPE) is obtained, the particles can be inert to the Li+ conduction (Al2O3, TiO2, SiO2, MgO, zeolite, montmorillonite, ...),[49][50][51] with the sole purpose of reducing the cristallinity, or active (LLTO, LLZO, LATP...)[52][53] if ISE's particles are dispersed and depending on the polymer/inorganic ratio the nomenclature ceramic-in-polymer and polymer-in-ceramic is often used.[54] Copolymerization,[55] crosslinking,[56] interpenetration,[57] and blending[58] may also be used as polymer/polymer coordination to tune the properties of the SPEs and achieve better performances, introducing in the polymeric chains polar groups like ethers, carbonyls or nitriles drastically improve the dissolution of the lithium salts.
Quasi-solid-state electrolyte
Often confused with SPEs, quasi-solid-state electrolytes (QSSEs) are also called gel polymer electrolytes (GPEs), but they have a substantially different ionic conduction mechanism: SPEs conducts ions through the interaction with the susbtitutional groups of the polymer chains, while GPEs conducts ions mainly in the solvent or plasticizer.[59] They consist of a polymer network swollen in a solvent that contains the active ions, so it possesses both the mechanical properties of solids and the high transport properties of liquids. Several GPEs with a number of polymer hosts have been studied, by using the same polymers as SPEs (e.g. PEO, PAN, PMMA, PVDF-HFP, ...), but synthetized with increased porosity in order to easily allocate a low-evaporation solvents like ethylene carbonate (EC), propylene carbonate (PC), diethyl carbonate (DEC), dimethyl carbonate (DMC), used as plasticizers.[60][61][62] Low molecular weight poly(ethylene glycol) (PEG) or other ethers or aprotic organic solvents with high dielectric constant like dimethylsulfoxide (DMSO) can also be mixed the SPE matrix.[63][64] UV and thermal crosslinking are useful ways to polymerize in-situ the GPE directly in contact with the electrodes for a perfectly adherent interface.[65] Values of ionic conductivity of few mS cm−1 can be easily achieved with GPEs, as demonstrate the numerous research articles published on the argument.[66]
Opportunities
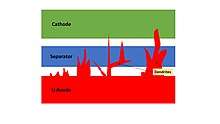
The versatility and properties of the solid-state electrolyte widen the possible applications towards high energy density and cheaper battery chemistries that are otherwise prevented by the current state-of-the-art of Li-ion batteries. Indeed, by introducing a SSE in the battery architecture there's the possibility to use metallic lithium as anode material, with the possibility to achieve a high energy density battery thanks to its high specific capacity of 3860 mAh g−1.[67] The utilization of a lithium metal anode (LMA) is prevented in a liquid electrolyte above all because of the dendritic growth of a pure Li electrode that easily cause short circuits after few cycles; other related issues are volume expansions, solid-electrolyte-interface (SEI) reactivity and 'dead' lithium.[68] The usage of a SSE guarantees a homogeneous contact with the metallic lithium electrode and possess the mechanical properties to impede the uncontrolled deposition of Li+ ions during the charging phase. At the same time, a SSE finds very promising application in lithium-sulfur batteries solving the key issue of the polysulfide "shuttle" effect by blocking the dissolution of polysulfide species in the electrolyte that rapidly causes a reduction of capacity.[69]
See also
- Solid-state battery
- Li-ion battery
- Lithium-sulfur battery
- Research in lithium-ion batteries
References
- "Japanese Government Partners With Manufacturers On Solid State Battery Research". CleanTechnica. 7 May 2018.
- "German Federal Government Invests In Solid State Battery Research". CleanTechnica. 29 October 2018.
- Chen, Zhen; Kim, Guk-Tae; Wang, Zeli; Bresser, Dominic; Qin, Bingsheng; Geiger, Dorin; Kaiser, Ute; Wang, Xuesen; Shen, Ze Xiang; Passerini, Stefano (October 2019). "4-V flexible all-solid-state lithium polymer batteries". Nano Energy. 64: 103986. doi:10.1016/j.nanoen.2019.103986.
- Wang, Renheng; Cui, Weisheng; Chu, Fulu; Wu, Feixiang (September 2020). "Lithium metal anodes: Present and future". Journal of Energy Chemistry. 48: 145–159. doi:10.1016/j.jechem.2019.12.024.
- Baldwin, Roberto (12 March 2020). "Samsung Reveals Breakthrough: Solid-State EV Battery with 500-Mile Range". Car and Driver.
- Kim, Taehoon; Song, Wentao; Son, Dae-Yong; Ono, Luis K.; Qi, Yabing (2019). "Lithium-ion batteries: outlook on present, future, and hybridized technologies". Journal of Materials Chemistry A. 7 (7): 2942–2964. doi:10.1039/c8ta10513h.
- "Solid-State Batteries". FutureBridge. 6 July 2019.
- Solid State Electrochemistry. Cambridge University Press. ISBN 9780511524790.
- Wright, Peter V. (September 1975). "Electrical conductivity in ionic complexes of poly(ethylene oxide)". British Polymer Journal. 7 (5): 319–327. doi:10.1002/pi.4980070505.
- GRAY, F; MACCALLUM, J; VINCENT, C (January 1986). "Poly(ethylene oxide) - LiCF3SO3 - polystyrene electrolyte systems". Solid State Ionics. 18-19: 282–286. doi:10.1016/0167-2738(86)90127-X.
- Janek, Jürgen; Zeier, Wolfgang G. (8 September 2016). "A solid future for battery development". Nature Energy. 1 (9): 16141. Bibcode:2016NatEn...116141J. doi:10.1038/nenergy.2016.141.
- Lee, Yong-Gun; Fujiki, Satoshi; Jung, Changhoon; Suzuki, Naoki; Yashiro, Nobuyoshi; Omoda, Ryo; Ko, Dong-Su; Shiratsuchi, Tomoyuki; Sugimoto, Toshinori; Ryu, Saebom; Ku, Jun Hwan; Watanabe, Taku; Park, Youngsin; Aihara, Yuichi; Im, Dongmin; Han, In Taek (9 March 2020). "High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes". Nature Energy. 5 (4): 299–308. Bibcode:2020NatEn...5..299L. doi:10.1038/s41560-020-0575-z.
- Agrawal, R C; Pandey, G P (21 November 2008). "Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview". Journal of Physics D: Applied Physics. 41 (22): 223001. doi:10.1088/0022-3727/41/22/223001.
- Sundaramahalingam, K.; Muthuvinayagam, M.; Nallamuthu, N.; Vanitha, D.; Vahini, M. (1 January 2019). "Investigations on lithium acetate-doped PVA/PVP solid polymer blend electrolytes". Polymer Bulletin. 76 (11): 5577–5602. doi:10.1007/s00289-018-02670-2. S2CID 104442538.
- Appetecchi, G. B. (1996). "A New Class of Advanced Polymer Electrolytes and Their Relevance in Plastic-like, Rechargeable Lithium Batteries". Journal of the Electrochemical Society. 143 (1): 6. Bibcode:1996JElS..143....6A. doi:10.1149/1.1836379.
- Zheng, Feng; Kotobuki, Masashi; Song, Shufeng; Lai, Man On; Lu, Li (June 2018). "Review on solid electrolytes for all-solid-state lithium-ion batteries". Journal of Power Sources. 389: 198–213. Bibcode:2018JPS...389..198Z. doi:10.1016/j.jpowsour.2018.04.022.
- Agostini, Marco; Lim, Du Hyun; Sadd, Matthew; Fasciani, Chiara; Navarra, Maria Assunta; Panero, Stefania; Brutti, Sergio; Matic, Aleksandar; Scrosati, Bruno (11 September 2017). "Stabilizing the Performance of High-Capacity Sulfur Composite Electrodes by a New Gel Polymer Electrolyte Configuration". ChemSusChem. 10 (17): 3490–3496. doi:10.1002/cssc.201700977. PMID 28731629.
- Mindemark, Jonas; Lacey, Matthew J.; Bowden, Tim; Brandell, Daniel (June 2018). "Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes". Progress in Polymer Science. 81: 114–143. doi:10.1016/j.progpolymsci.2017.12.004.
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. (June 2017). "Challenges and issues facing lithium metal for solid-state rechargeable batteries" (PDF). Journal of Power Sources. 353: 333–342. Bibcode:2017JPS...353..333M. doi:10.1016/j.jpowsour.2017.04.018.
- Bachman, John Christopher; Muy, Sokseiha; Grimaud, Alexis; Chang, Hao-Hsun; Pour, Nir; Lux, Simon F.; Paschos, Odysseas; Maglia, Filippo; Lupart, Saskia; Lamp, Peter; Giordano, Livia; Shao-Horn, Yang (29 December 2015). "Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction". Chemical Reviews. 116 (1): 140–162. doi:10.1021/acs.chemrev.5b00563. hdl:1721.1/109539. PMID 26713396.
- Zhao, Qing; Stalin, Sanjuna; Zhao, Chen-Zi; Archer, Lynden A. (5 February 2020). "Designing solid-state electrolytes for safe, energy-dense batteries". Nature Reviews Materials. 5 (3): 229–252. Bibcode:2020NatRM...5..229Z. doi:10.1038/s41578-019-0165-5. S2CID 211028485.
- Han, Xiaogang; Gong, Yunhui; Fu, Kun (Kelvin); He, Xingfeng; Hitz, Gregory T.; Dai, Jiaqi; Pearse, Alex; Liu, Boyang; Wang, Howard; Rubloff, Gary; Mo, Yifei; Thangadurai, Venkataraman; Wachsman, Eric D.; Hu, Liangbing (19 December 2016). "Negating interfacial impedance in garnet-based solid-state Li metal batteries". Nature Materials. 16 (5): 572–579. doi:10.1038/nmat4821. OSTI 1433807. PMID 27992420.
- Kraft, Marvin A.; Ohno, Saneyuki; Zinkevich, Tatiana; Koerver, Raimund; Culver, Sean P.; Fuchs, Till; Senyshyn, Anatoliy; Indris, Sylvio; Morgan, Benjamin J.; Zeier, Wolfgang G. (November 2018). "Inducing High Ionic Conductivity in the Lithium Superionic Argyrodites Li P Ge S I for All-Solid-State Batteries". Journal of the American Chemical Society. 140 (47): 16330–16339. doi:10.1021/jacs.8b10282. PMID 30380843.
- Liu, Qi; Geng, Zhen; Han, Cuiping; Fu, Yongzhu; Li, Song; He, Yan-bing; Kang, Feiyu; Li, Baohua (June 2018). "Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries". Journal of Power Sources. 389: 120–134. Bibcode:2018JPS...389..120L. doi:10.1016/j.jpowsour.2018.04.019.
- DeWees, Rachel; Wang, Hui (24 July 2019). "Synthesis and Properties of NaSICON‐type LATP and LAGP Solid Electrolytes". ChemSusChem. 12 (16): 3713–3725. doi:10.1002/cssc.201900725. PMID 31132230.
- Beister, Heinz Jürgen; Haag, Sabine; Kniep, Rüdiger; Strössner, Klaus; Syassen, Karl (August 1988). "Phase Transformations of Lithium Nitride under Pressure". Angewandte Chemie International Edition in English. 27 (8): 1101–1103. doi:10.1002/anie.198811011.
- de Jongh, P. E.; Blanchard, D.; Matsuo, M.; Udovic, T. J.; Orimo, S. (3 March 2016). "Complex hydrides as room-temperature solid electrolytes for rechargeable batteries". Applied Physics A. 122 (3): 251. Bibcode:2016ApPhA.122..251D. doi:10.1007/s00339-016-9807-2. S2CID 53402745.
- Li, Yutao; Xu, Henghui; Chien, Po-Hsiu; Wu, Nan; Xin, Sen; Xue, Leigang; Park, Kyusung; Hu, Yan-Yan; Goodenough, John B. (9 July 2018). "A Perovskite Electrolyte That Is Stable in Moist Air for Lithium-Ion Batteries". Angewandte Chemie International Edition. 57 (28): 8587–8591. doi:10.1002/anie.201804114. PMID 29734500.
- Asano, Tetsuya; Sakai, Akihiro; Ouchi, Satoru; Sakaida, Masashi; Miyazaki, Akinobu; Hasegawa, Shinya (November 2018). "Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries". Advanced Materials. 30 (44): 1803075. doi:10.1002/adma.201803075. PMID 30216562.
- Senevirathne, Keerthi; Day, Cynthia S.; Gross, Michael D.; Lachgar, Abdessadek; Holzwarth, N.A.W. (February 2013). "A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure". Solid State Ionics. 233: 95–101. doi:10.1016/j.ssi.2012.12.013.
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. (4 April 2005). "New, Highly Ion-Conductive Crystals Precipitated from Li2S-P2S5 Glasses". Advanced Materials. 17 (7): 918–921. doi:10.1002/adma.200401286.
- Mindemark, Jonas; Lacey, Matthew J.; Bowden, Tim; Brandell, Daniel (June 2018). "Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes". Progress in Polymer Science. 81: 114–143. doi:10.1016/j.progpolymsci.2017.12.004.
- Hallinan, Daniel T.; Balsara, Nitash P. (July 2013). "Polymer Electrolytes". Annual Review of Materials Research. 43 (1): 503–525. Bibcode:2013AnRMS..43..503H. doi:10.1146/annurev-matsci-071312-121705.
- Manuel Stephan, A.; Nahm, K.S. (July 2006). "Review on composite polymer electrolytes for lithium batteries". Polymer. 47 (16): 5952–5964. doi:10.1016/j.polymer.2006.05.069.
- Fenton, D.E.; Parker, J.M.; Wright, P.V. (November 1973). "Complexes of alkali metal ions with poly(ethylene oxide)". Polymer. 14 (11): 589. doi:10.1016/0032-3861(73)90146-8.
- Payne, D.R.; Wright, P.V. (May 1982). "Morphology and ionic conductivity of some lithium ion complexes with poly(ethylene oxide)". Polymer. 23 (5): 690–693. doi:10.1016/0032-3861(82)90052-0.
- Sun, Bing; Mindemark, Jonas; Edström, Kristina; Brandell, Daniel (September 2014). "Polycarbonate-based solid polymer electrolytes for Li-ion batteries". Solid State Ionics. 262: 738–742. doi:10.1016/j.ssi.2013.08.014.
- Webb, Michael A.; Jung, Yukyung; Pesko, Danielle M.; Savoie, Brett M.; Yamamoto, Umi; Coates, Geoffrey W.; Balsara, Nitash P.; Wang, Zhen-Gang; Miller, Thomas F. (10 July 2015). "Systematic Computational and Experimental Investigation of Lithium-Ion Transport Mechanisms in Polyester-Based Polymer Electrolytes". ACS Central Science. 1 (4): 198–205. doi:10.1021/acscentsci.5b00195. PMC 4827473. PMID 27162971.
- Hu, Pu; Chai, Jingchao; Duan, Yulong; Liu, Zhihong; Cui, Guanglei; Chen, Liquan (2016). "Progress in nitrile-based polymer electrolytes for high performance lithium batteries". Journal of Materials Chemistry A. 4 (26): 10070–10083. doi:10.1039/C6TA02907H.
- Mindemark, Jonas; Sun, Bing; Törmä, Erik; Brandell, Daniel (December 2015). "High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature". Journal of Power Sources. 298: 166–170. Bibcode:2015JPS...298..166M. doi:10.1016/j.jpowsour.2015.08.035.
- Zhang, Lei; Wang, Shi; Li, Jingyu; Liu, Xu; Chen, Pingping; Zhao, Tong; Zhang, Liaoyun (2019). "A nitrogen-containing all-solid-state hyperbranched polymer electrolyte for superior performance lithium batteries". Journal of Materials Chemistry A. 7 (12): 6801–6808. doi:10.1039/C9TA00180H.
- Wang, Qinglei; Zhang, Huanrui; Cui, Zili; Zhou, Qian; Shangguan, Xuehui; Tian, Songwei; Zhou, Xinhong; Cui, Guanglei (December 2019). "Siloxane-based polymer electrolytes for solid-state lithium batteries". Energy Storage Materials. 23: 466–490. doi:10.1016/j.ensm.2019.04.016.
- Rohan, Rupesh; Pareek, Kapil; Chen, Zhongxin; Cai, Weiwei; Zhang, Yunfeng; Xu, Guodong; Gao, Zhiqiang; Cheng, Hansong (2015). "A high performance polysiloxane-based single ion conducting polymeric electrolyte membrane for application in lithium ion batteries". Journal of Materials Chemistry A. 3 (40): 20267–20276. doi:10.1039/c5ta02628h.
- Jacob, M (11 December 1997). "Effect of PEO addition on the electrolytic and thermal properties of PVDF-LiClO4 polymer electrolytes". Solid State Ionics. 104 (3–4): 267–276. doi:10.1016/S0167-2738(97)00422-0.
- Liu, Bo; Huang, Yun; Cao, Haijun; Song, Amin; Lin, Yuanhua; Wang, Mingshan; Li, Xing (28 October 2017). "A high-performance and environment-friendly gel polymer electrolyte for lithium ion battery based on composited lignin membrane". Journal of Solid State Electrochemistry. 22 (3): 807–816. doi:10.1007/s10008-017-3814-x. S2CID 103666062.
- Yahya, M.Z.A.; Arof, A.K. (May 2003). "Effect of oleic acid plasticizer on chitosan–lithium acetate solid polymer electrolytes". European Polymer Journal. 39 (5): 897–902. doi:10.1016/S0014-3057(02)00355-5.
- Zhao, Lingzhu; Fu, Jingchuan; Du, Zhi; Jia, Xiaobo; Qu, Yanyu; Yu, Feng; Du, Jie; Chen, Yong (January 2020). "High-strength and flexible cellulose/PEG based gel polymer electrolyte with high performance for lithium ion batteries". Journal of Membrane Science. 593: 117428. doi:10.1016/j.memsci.2019.117428.
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. (September 1983). "Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts". Solid State Ionics. 11 (1): 91–95. doi:10.1016/0167-2738(83)90068-1.
- Lin, Dingchang; Liu, Wei; Liu, Yayuan; Lee, Hye Ryoung; Hsu, Po-Chun; Liu, Kai; Cui, Yi (December 2015). "High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO Nanospheres in Poly(ethylene oxide)". Nano Letters. 16 (1): 459–465. doi:10.1021/acs.nanolett.5b04117. PMID 26595277.
- Kumar, B (2 September 1999). "Polymer ceramic composite electrolytes: conductivity and thermal history effects". Solid State Ionics. 124 (3–4): 239–254. doi:10.1016/S0167-2738(99)00148-4.
- Kumar, Binod; Scanlon, Lawrence; Marsh, Richard; Mason, Rachel; Higgins, Robert; Baldwin, Richard (March 2001). "Structural evolution and conductivity of PEO:LiBF4–MgO composite electrolytes". Electrochimica Acta. 46 (10–11): 1515–1521. doi:10.1016/S0013-4686(00)00747-7.
- Liang, Xinghua; Han, Di; Wang, Yunting; Lan, Lingxiao; Mao, Jie (2018). "Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries". RSC Advances. 8 (71): 40498–40504. doi:10.1039/C8RA08436J.
- Keller, Marlou; Appetecchi, Giovanni Battista; Kim, Guk-Tae; Sharova, Varvara; Schneider, Meike; Schuhmacher, Jörg; Roters, Andreas; Passerini, Stefano (June 2017). "Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li 7 La 3 Zr 2 O 12 in P(EO) 15 LiTFSI". Journal of Power Sources. 353: 287–297. Bibcode:2017JPS...353..287K. doi:10.1016/j.jpowsour.2017.04.014.
- Chen, Long; Li, Yutao; Li, Shuai-Peng; Fan, Li-Zhen; Nan, Ce-Wen; Goodenough, John B. (April 2018). "PEO/garnet composite electrolytes for solid-state lithium batteries: From "ceramic-in-polymer" to "polymer-in-ceramic"". Nano Energy. 46: 176–184. doi:10.1016/j.nanoen.2017.12.037.
- Bouchet, Renaud; Maria, Sébastien; Meziane, Rachid; Aboulaich, Abdelmaula; Lienafa, Livie; Bonnet, Jean-Pierre; Phan, Trang N. T.; Bertin, Denis; Gigmes, Didier; Devaux, Didier; Denoyel, Renaud; Armand, Michel (31 March 2013). "Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries". Nature Materials. 12 (5): 452–457. Bibcode:2013NatMa..12..452B. doi:10.1038/nmat3602. PMID 23542871.
- Zhang, Yuhang; Lu, Wei; Cong, Lina; Liu, Jia; Sun, Liqun; Mauger, Alain; Julien, Christian M.; Xie, Haiming; Liu, Jun (April 2019). "Cross-linking network based on Poly(ethylene oxide): Solid polymer electrolyte for room temperature lithium battery". Journal of Power Sources. 420: 63–72. Bibcode:2019JPS...420...63Z. doi:10.1016/j.jpowsour.2019.02.090.
- Liu, Xiaochen; Ding, Guoliang; Zhou, Xinhong; Li, Shizhen; He, Weisheng; Chai, Jingchao; Pang, Chunguang; Liu, Zhihong; Cui, Guanglei (2017). "An interpenetrating network poly(diethylene glycol carbonate)-based polymer electrolyte for solid state lithium batteries". Journal of Materials Chemistry A. 5 (22): 11124–11130. doi:10.1039/C7TA02423A.
- Rajendran, S; Sivakumar, M; Subadevi, R (February 2004). "Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes". Materials Letters. 58 (5): 641–649. doi:10.1016/S0167-577X(03)00585-8.
- Liang, Shishuo; Yan, Wenqi; Wu, Xu; Zhang, Yi; Zhu, Yusong; Wang, Hongwei; Wu, Yuping (May 2018). "Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance". Solid State Ionics. 318: 2–18. doi:10.1016/j.ssi.2017.12.023.
- Lithium batteries : new materials, developments, and perspectives. Elsevier. ISBN 9780444899576.
- Watanabe, Masayoshi; Kanba, Motoi; Nagaoka, Katsuro; Shinohara, Isao (November 1982). "Ionic conductivity of hybrid films based on polyacrylonitrile and their battery application". Journal of Applied Polymer Science. 27 (11): 4191–4198. doi:10.1002/app.1982.070271110.
- Appetecchi, G.B.; Croce, F.; Scrosati, B. (June 1995). "Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes". Electrochimica Acta. 40 (8): 991–997. doi:10.1016/0013-4686(94)00345-2.
- Ahmed, Hawzhin T.; Jalal, Viyan J.; Tahir, Dana A.; Mohamad, Azhin H.; Abdullah, Omed Gh. (December 2019). "Effect of PEG as a plasticizer on the electrical and optical properties of polymer blend electrolyte MC-CH-LiBF4 based films". Results in Physics. 15: 102735. Bibcode:2019ResPh..1502735A. doi:10.1016/j.rinp.2019.102735.
- Verdier, Nina; Lepage, David; Zidani, Ramzi; Prébé, Arnaud; Aymé-Perrot, David; Pellerin, Christian; Dollé, Mickaël; Rochefort, Dominic (27 December 2019). "Cross-Linked Polyacrylonitrile-Based Elastomer Used as Gel Polymer Electrolyte in Li-Ion Battery". ACS Applied Energy Materials. 3 (1): 1099–1110. doi:10.1021/acsaem.9b02129.
- Gerbaldi, C.; Nair, J.R.; Meligrana, G.; Bongiovanni, R.; Bodoardo, S.; Penazzi, N. (January 2010). "UV-curable siloxane-acrylate gel-copolymer electrolytes for lithium-based battery applications". Electrochimica Acta. 55 (4): 1460–1467. doi:10.1016/j.electacta.2009.05.055.
- Bi, Haitao; Sui, Gang; Yang, Xiaoping (December 2014). "Studies on polymer nanofibre membranes with optimized core–shell structure as outstanding performance skeleton materials in gel polymer electrolytes". Journal of Power Sources. 267: 309–315. Bibcode:2014JPS...267..309B. doi:10.1016/j.jpowsour.2014.05.030.
- Yuan, Huadong; Nai, Jianwei; Tian, He; Ju, Zhijin; Zhang, Wenkui; Liu, Yujing; Tao, Xinyong; Lou, Xiong Wen (David) (6 March 2020). "An ultrastable lithium metal anode enabled by designed metal fluoride spansules". Science Advances. 6 (10): eaaz3112. Bibcode:2020SciA....6.3112Y. doi:10.1126/sciadv.aaz3112. PMID 32181364. S2CID 212739571.
- Li, Linlin; Li, Siyuan; Lu, Yingying (2018). "Suppression of dendritic lithium growth in lithium metal-based batteries". Chemical Communications. 54 (50): 6648–6661. doi:10.1039/C8CC02280A. PMID 29796542.
- Long, Canghai; Li, Libo; Zhai, Mo; Shan, Yuhang (November 2019). "Facile preparation and electrochemistry performance of quasi solid-state polymer lithium–sulfur battery with high-safety and weak shuttle effect". Journal of Physics and Chemistry of Solids. 134: 255–261. Bibcode:2019JPCS..134..255L. doi:10.1016/j.jpcs.2019.06.017.
External links
- Solid-state battery. Retrieved 2020-06-26.