Solenopsin
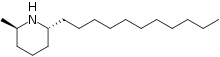
Solenopsin is an alkaloid with the molecular formula C17H35N found in the venom of fire ants (Solenopsis). It is considered the primary toxin in the venom[2] and may be the component responsible for the cardiorespiratory failure in people who experience excessive fire ant stings.[3]
 | |
| Names | |
|---|---|
| IUPAC name
(2R,6R)-2-Methyl-6-undecylpiperidine[1] | |
| Other names
Solenopsin A | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H35N | |
| Molar mass | 253.474 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structurally solenopsins are a piperidine ring with a methyl group substitution at position 2 and a long hydrophobic chain at position 6. They are typically oily at room temperature, water-insoluble, and present an absorbance peak at 232 nanometers.[4] Fire ant venom contains other chemically related piperidines which make purification of solenopsin from ants difficult.[5][6] Therefore, solenopsin and related compounds have been the target of organic synthesis from which pure compounds can be produced for individual study. Originally synthesized in 1998, several groups have designed novel and creative methods of synthesizing enantiopure solenopsin and other alkaloidal components of ant venom.
Total synthesis
The total synthesis of solenopsin has been described by several methods.[7] A proposed method of synthesis [8](Figure 1) starts with alkylation of 4-chloropyridine with a Grignard reagent derived from 1-bromoundecane, followed by reaction with phenyl chloroformate to form 4-chloro-1-(phenoxycarbonyl)-2-n-undecyl-1,2-dihydropyridine. The phenylcarbamate is converted to the BOC protecting group, and then pyridine is methylated at the 6 position. The pyridine ring is then reduced to a tetrahydropyridine via catalytic hydrogenation with Pd/C and then further reduced with sodium cyanoborohydride to a piperidine ring. The BOC group is finally removed to yield solenopsin. A number of analogs have been synthesized using modifications of this procedure.
A shorter method of synthesis stemming from commercially-available lutidine has been more recently proposed.[9]
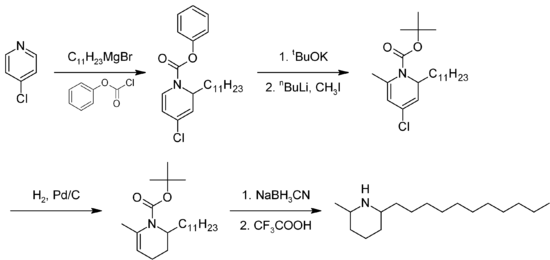 Figure 1. Example synthesis of racemic solenopsin
Figure 1. Example synthesis of racemic solenopsin
Biological activities
Solenopsins are described as toxic against vertebrates and invertebrates. For example, the compound known as isosolenopsin A has been demonstrated to have strong insecticidal effects [10] which may play a central role in the biology of fire ants.
In addition to its toxicity, solenopsis has a number of other biological activities. It inhibits angiogenesis in vitro via the phosphoinositide 3-kinase (PI3K) signaling pathway,[8] inhibits neuronal nitric oxide synthase (nNOS) in a manner that appears to be non-competitive with L-arginine,[11] and inhibits quorum-sensing signaling in some bacteria.[12] The biological activities of solenopsins have led researchers to propose a number of biotechnological and biomedical applications for these compounds. For instance, mentioned anti-bacterial and interference in quorum-sensing signalling apparently provide solenopsins with considerable anti-biofilm activity, which suggests the potential of analogs as new disinfectants and surface-conditioning agents.[13] Also, solenopsins have been demonstrated to inhibit cell division and viability of Trypanosoma cruzi, the cause of Chaga's disease, which suggests these alkaloids as potential chemotherapeutic drugs.[14]
Solenopsin and analogs share structural and biological properties with the sphingolipid ceramide, a major endogenous regulator of cell signaling, inducing mitophagy and anti-proliferative effects in different tumor cell lines.[15]
Synthetic analogs of solenopsin are being studied for the potential treatment of psoriasis.[16]
References
- Stereochemistry per: Leclercq, S.; Thirionet, I.; Broeders, F.; Daloze, D.; Vander Meer, R.; Braekman, J.C. (1994). "Absolute configuration of the solenopsins, venom alkaloids of the fire ants". Tetrahedron. 50 (28): 8465–8478. doi:10.1016/S0040-4020(01)85567-8.
- Touchard, A; Aili, S. R; Fox, E. G; Escoubas, P; Orivel, J; Nicholson, G. M; Dejean, A (2016). "The Biochemical Toxin Arsenal from Ant Venoms". Toxins. 8 (1): 30. doi:10.3390/toxins8010030. PMC 4728552. PMID 26805882.
- Howell G, Butler J, Deshazo RD, Farley JM, Liu HL, Nanayakkara NP, Yates A, Yi GB, Rockhold RW (2005). "Cardiodepressant and neurologic actions of Solenopsis invicta (imported fire ant) venom alkaloids". Ann Allergy Asthma Immunol. 94 (3): 380–6. doi:10.1016/S1081-1206(10)60991-X. PMID 15801250.
- Fox, Eduardo G.P.; Xu, Meng; Wang, Lei; Chen, Li; Lu, Yong-Yue (June 2018). "Gas-chromatography and UV-spectroscopy of Hymenoptera venoms obtained by trivial centrifugation". Data in Brief. 18: 992–998. doi:10.1016/j.dib.2018.03.101. PMC 5996826. PMID 29900266.
- Gopalakrishnakone, P.; Calvete, Juan J. (2021-01-14). Venom genomics and proteomics. Gopalakrishnakone, P.,, Calvete, Juan J. (Living Reference Work ed.). Dordrecht. ISBN 9789400766495. OCLC 968345667.
- Fox, Eduardo G.P.; Xu, Meng; Wang, Lei; Chen, Li; Lu, Yong-Yue (May 2018). "Speedy milking of fresh venom from aculeate hymenopterans". Toxicon. 146: 120–123. doi:10.1016/j.toxicon.2018.02.050. PMID 29510162.
- Leclercq, S.; Daloze, D.; Braekman, J.-C. (1996). "A Synthesis of the Fire Ant Alkaloids, Solenopsins". Org. Prep. Proced. Int. 28 (5): 499. doi:10.1080/00304949609458571. Archived from the original on 2003-03-20.
- Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, Vlahos CJ, Ye K, Perry BN, Matter W, Fischl A, Cook J, Silver PA, Bain J, Cohen P, Whitmire D, Furness S, Govindarajan B, Bowen JP (2007). "Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis". Blood. 109 (2): 560–5. doi:10.1182/blood-2006-06-029934. PMC 1785094. PMID 16990598.
- Pianaro, Adriana; Fox, Eduardo G.P.; Bueno, Odair C.; Marsaioli, Anita J. (May 2012). "Rapid configuration analysis of the solenopsins". Tetrahedron: Asymmetry. 23 (9): 635–642. doi:10.1016/j.tetasy.2012.05.005.
- Fox, Eduardo G.P.; Wu, Xiaoqing; Wang, Lei; Chen, Li; Lu, Yong-Yue; Xu, Yijuan (February 2019). "Queen venom isosolenopsin A delivers rapid incapacitation of fire ant competitors". Toxicon. 158: 77–83. doi:10.1016/j.toxicon.2018.11.428. PMID 30529381.
- Yi GB, McClendon D, Desaiah D, Goddard J, Lister A, Moffitt J, Meer RK, deShazo R, Lee KS, Rockhold RW (2003). "Fire ant venom alkaloid, isosolenopsin A, a potent and selective inhibitor of neuronal nitric oxide synthase". Int J Toxicol. 22 (2): 81–6. doi:10.1080/10915810305090. PMID 12745988.
- Park, Junguk; Kaufmann, Gunnar F; Bowen, J. Phillip; Arbiser, Jack L; Janda, Kim D (2008). "Solenopsin A, a Venom Alkaloid from the Fire Ant Solenopsis invicta,Inhibits Quorum‐Sensing Signaling in Pseudomonas aeruginosa". The Journal of Infectious Diseases. 198 (8): 1198–201. doi:10.1086/591916. PMID 18713055.
- Machado, Ednildo de Alcântara; Castilho, Livia Vieira Araujo de; Domont, Gilberto B.; Nogueira, Fabio C. S.; Freire, Denise Maria Guimarães; Sousa, Joab Sampaio de; Santos, Diogo Gama dos; Fox, Eduardo Gonçalves Paterson; Carvalho, Danielle Bruno de (July 2019). "Fire Ant Venom Alkaloids Inhibit Biofilm Formation". Toxins. 11 (7): 420. doi:10.3390/toxins11070420. PMID 31323790.
- Silva, Rafael C. M. Costa; Fox, Eduardo G. P.; Gomes, Fabio M.; Feijó, Daniel F.; Ramos, Isabela; Koeller, Carolina M.; Costa, Tatiana F. R.; Rodrigues, Nathalia S.; Lima, Ana P.; Atella, Georgia C.; Miranda, Kildare (December 2020). "Venom alkaloids against Chagas disease parasite: search for effective therapies". Scientific Reports. 10 (1): 10642. doi:10.1038/s41598-020-67324-8. ISSN 2045-2322.
- Karlsson I, Zhou X, Thomas R, Smith AT, Bonner MY, Bakshi P, Banga AK, Bowen JP, Qabaja G, Ford SL, Ballard MD, Petersen KS, Li X, Chen G, Ogretmen B, Zhang J, Watkins EB, Arnold RS, Arbiser J (2015). "Solenopsin A and analogs exhibit ceramide-like biological activity". Vascular Cell. 7 (5): 5. doi:10.1186/s13221-015-0030-2. PMC 4443652. PMID 26015865.
- Arbiser, Jack L; Nowak, Ron; Michaels, Kellie; Skabytska, Yuliya; Biedermann, Tilo; Lewis, Monica J; Bonner, Michael Y; Rao, Shikha; Gilbert, Linda C; Yusuf, Nabiha; Karlsson, Isabella; Fritz, Yi; Ward, Nicole L (2017). "Evidence for biochemical barrier restoration: Topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis". Scientific Reports. 7 (1): 11198. Bibcode:2017NatSR...711198A. doi:10.1038/s41598-017-10580-y. PMC 5593857. PMID 28894119.
Further reading
- O'Hagan, David (1997). "Pyrrole, pyrrolidine pyridine, piperidine, azepine and tropane alkaloids". Natural Product Reports (Review). 14 (6): 637. doi:10.1039/NP9971400637.