Shibasaki catalysts
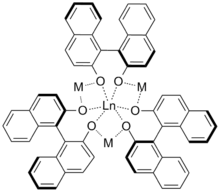
Shibasaki catalysts are a class of hetero-bimetallic complexes with the general formula [Ln(binol)3(M)3] (M = alkali metal, Ln = lanthanide). They are named after Masakatsu Shibasaki, whose group first developed them, and are used as asymmetric catalysts.
Development
The Shibasaki group produced the first chiral lanthanide-binaphtholate complex in 1992, which was used to catalyse nitroaldol reactions.[1] The complex was not characterised but was the first to perform the reaction enantioselectively. This success led to further research which resulted in the development of heterometallic complexes with the formula [Ln(binol)3(M)3], the structure of which was elucidated by X-ray crystallography.[2]
Scope
Shibasaki catalysts are effective for a wide range of enantioselective reactions including nitroaldol,[3] Michael,[4] Diels-Alder[5] and hydrophosphonylation reactions.[6] Their effectiveness arises in part from their ability to act as both a Brønsted base by virtue of the metal alkoxide and a Lewis acid via the lanthanide ion. Enantioselectivity has been found to be sensitive to both Ln and M; with the nitroaldol reaction being most effective when Ln = Eu and M = Li[7] whereas the Michael reaction requires Ln = La and M = Na.[8] It was observed that alterations of Ln and M caused predictable changes in the bite angle of the binaphthol backbone.
References
- Sasai, Hiroaki; Suzuki, Takeyuki; Arai, Shigeru; Arai, Takayoshi; Shibasaki, Masakatsu (1 May 1992). "Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions". Journal of the American Chemical Society. 114 (11): 4418–4420. doi:10.1021/ja00037a068.
- Sasai, Hiroaki; Suzuki, Takeyuki; Itoh, Noriie; Tanaka, Koichi; Date, Tadamasa; Okamura, Kimio; Shibasaki, Masakatsu (1 November 1993). "Catalytic asymmetric nitroaldol reaction using optically active rare earth BINOL complexes: investigation of the catalyst structure". Journal of the American Chemical Society. 115 (22): 10372–10373. doi:10.1021/ja00075a068.
- Sasai, Hiroaki; Kim, Won-Sup; Suzuki, Takeyuki; Shibasaki, Masakatsu; Mitsuda, Masaru; Hasegawa, Junzo; Ohashi, Takehisa (1994). "Diastereoselective catalytic asymmetric nitroaldol reaction utilizing rare earth-Li-(R)-BINOL complex. A highly efficient synthesis of norstatine". Tetrahedron Letters. 35 (33): 6123–6126. doi:10.1016/0040-4039(94)88093-X.
- Sasai, Hiroaki; Arai, Takayoshi; Satow, Yoshinori; Houk, K. N.; Shibasaki, Masakatsu (1 June 1995). "The First Heterobimetallic Multifunctional Asymmetric Catalyst". Journal of the American Chemical Society. 117 (23): 6194–6198. doi:10.1021/ja00128a005.
- Morita, Takahiro; Arai, Takayoshi; Sasai, Hiroaki; Shibasaki, Masakatsu (1998). "Utilization of heterobimetallic complexes as Lewis acids". Tetrahedron: Asymmetry. 9 (8): 1445–1450. doi:10.1016/S0957-4166(98)00124-4.
- Sasai, Hiroaki; Arai, Shigeru; Tahara, Yoshihiro; Shibasaki, Masakatsu (1 October 1995). "Catalytic Asymmetric Synthesis of .alpha.-Amino Phosphonates Using Lanthanoid-Potassium-BINOL Complexes". The Journal of Organic Chemistry. 60 (21): 6656–6657. doi:10.1021/jo00126a003.
- Sasai, Hiroaki; Suzuki, Takeyuki; Itoh, Noriie; Arai, Shigeru; Shibasaki, Masakatsu (1993). "Effects of rare earth metals on the catalytic asymmetric nitroaldol reaction". Tetrahedron Letters. 34 (16): 2657–2660. doi:10.1016/S0040-4039(00)77649-0.
- Sasai, Hiroaki; Arai, Takayoshi; Satow, Yoshinori; Houk, K. N.; Shibasaki, Masakatsu (1 June 1995). "The First Heterobimetallic Multifunctional Asymmetric Catalyst". Journal of the American Chemical Society. 117 (23): 6194–6198. doi:10.1021/ja00128a005.