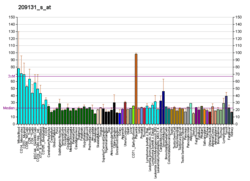
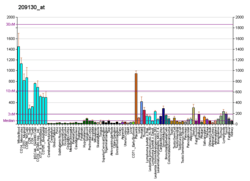
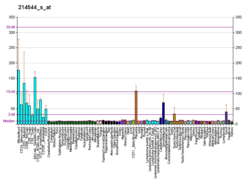
SNAP23
Synaptosomal-associated protein 23 is a protein that in humans is encoded by the SNAP23 gene.[5][6] Two alternative transcript variants encoding different protein isoforms have been described for this gene.
Function
Specificity of vesicular transport is regulated, in part, by the interaction of a vesicle-associated membrane protein termed synaptobrevin/VAMP with a target compartment membrane protein termed syntaxin. These proteins, together with SNAP25 (synaptosome-associated protein of 25 kDa), form a complex which serves as a binding site for the general membrane fusion machinery. Synaptobrevin/VAMP and syntaxin are believed to be involved in vesicular transport in most, if not all cells, while SNAP25 is present almost exclusively in the brain, suggesting that a ubiquitously expressed homolog of SNAP25 exists to facilitate transport vesicle/target membrane fusion in other tissues.
SNAP23 is structurally and functionally similar to SNAP25 and binds tightly to multiple syntaxins and synaptobrevins/VAMPs. It is an essential component of the high affinity receptor for the general membrane fusion machinery and is an important regulator of transport vesicle docking and fusion.[7]
Clinical significance
In individuals with insulin resistance, SNAP23 is found to be translocated from the plasma membrane to the cytosol where it becomes associated with lipid droplets and is therefore unable to translocate GLUT-4 to the membrane, hindering glucose transport.
Interactions
SNAP23 has been shown to interact with:
- Cystic fibrosis transmembrane conductance regulator,[8]
- KIF5B,[9]
- NAPA,[10]
- SNAPAP,[11]
- STX11,[10][12]
- STX1A,[6][13][14][15][16]
- STX2,[6][13][14][15]
- STX4,[6][13][14][15][17][18][16][19]
- STX6,[20]
- SYBL1,[21][22]
- Syntaxin 3,[6][13][15][16]
- VAMP2,[13][17][18]
- VAMP3,[13][17][19] and
- Vesicle-associated membrane protein 8.[13][17]
References
- GRCh38: Ensembl release 89: ENSG00000092531 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027287 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Mollinedo F, Lazo PA (Feb 1997). "Identification of two isoforms of the vesicle-membrane fusion protein SNAP-23 in human neutrophils and HL-60 cells". Biochemical and Biophysical Research Communications. 231 (3): 808–12. doi:10.1006/bbrc.1997.6196. PMID 9070898.
- Ravichandran V, Chawla A, Roche PA (Jun 1996). "Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues". The Journal of Biological Chemistry. 271 (23): 13300–3. doi:10.1074/jbc.271.23.13300. PMID 8663154.
- "Entrez Gene: SNAP23 synaptosomal-associated protein, 23kDa".
- Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL (Sep 2002). "CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex". Proceedings of the National Academy of Sciences of the United States of America. 99 (19): 12477–82. doi:10.1073/pnas.192203899. PMC 129470. PMID 12209004.
- Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL (Dec 2002). "The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23". Biochemistry. 41 (50): 14906–15. doi:10.1021/bi026417u. PMID 12475239.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (Oct 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E, Rowe T (Oct 2003). "Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells". The Biochemical Journal. 375 (Pt 2): 433–40. doi:10.1042/BJ20030427. PMC 1223698. PMID 12877659.
- Valdez AC, Cabaniols JP, Brown MJ, Roche PA (Mar 1999). "Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network". Journal of Cell Science. 112 (6): 845–54. PMID 10036234.
- Imai A, Nashida T, Yoshie S, Shimomura H (Aug 2003). "Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane". Archives of Oral Biology. 48 (8): 597–604. doi:10.1016/S0003-9969(03)00116-X. PMID 12828989.
- Li G, Alexander EA, Schwartz JH (May 2003). "Syntaxin isoform specificity in the regulation of renal H+-ATPase exocytosis". The Journal of Biological Chemistry. 278 (22): 19791–7. doi:10.1074/jbc.M212250200. PMID 12651853.
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M (May 1997). "Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c". Biochemical and Biophysical Research Communications. 234 (1): 257–62. doi:10.1006/bbrc.1997.6560. PMID 9168999.
- Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH (Dec 1998). "Three novel proteins of the syntaxin/SNAP-25 family". The Journal of Biological Chemistry. 273 (51): 34171–9. doi:10.1074/jbc.273.51.34171. PMID 9852078.
- Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M (Jun 2000). "Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment". Journal of Immunology. 164 (11): 5850–7. doi:10.4049/jimmunol.164.11.5850. PMID 10820264.
- Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M (Mar 2000). "Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2". The Journal of Biological Chemistry. 275 (11): 8240–7. doi:10.1074/jbc.275.11.8240. PMID 10713150.
- Freedman SJ, Song HK, Xu Y, Sun ZY, Eck MJ (Apr 2003). "Homotetrameric structure of the SNAP-23 N-terminal coiled-coil domain". The Journal of Biological Chemistry. 278 (15): 13462–7. doi:10.1074/jbc.M210483200. PMID 12556468.
- Martín-Martín B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F (Oct 2000). "Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis". Blood. 96 (7): 2574–83. PMID 11001914.
- Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, D'Esposito M, Galli T (Jul 2003). "A dual mechanism controlling the localization and function of exocytic v-SNAREs". Proceedings of the National Academy of Sciences of the United States of America. 100 (15): 9011–6. doi:10.1073/pnas.1431910100. PMC 166429. PMID 12853575.
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D (Jun 1998). "A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells". Molecular Biology of the Cell. 9 (6): 1437–48. doi:10.1091/mbc.9.6.1437. PMC 25366. PMID 9614185.
Further reading
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M (May 1997). "Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c". Biochemical and Biophysical Research Communications. 234 (1): 257–62. doi:10.1006/bbrc.1997.6560. PMID 9168999.
- Tang BL, Tan AE, Lim LK, Lee SS, Low DY, Hong W (Mar 1998). "Syntaxin 12, a member of the syntaxin family localized to the endosome". The Journal of Biological Chemistry. 273 (12): 6944–50. doi:10.1074/jbc.273.12.6944. PMID 9507000.
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D (Jun 1998). "A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells". Molecular Biology of the Cell. 9 (6): 1437–48. doi:10.1091/mbc.9.6.1437. PMC 25366. PMID 9614185.
- Foster LJ, Yeung B, Mohtashami M, Ross K, Trimble WS, Klip A (Aug 1998). "Binary interactions of the SNARE proteins syntaxin-4, SNAP23, and VAMP-2 and their regulation by phosphorylation". Biochemistry. 37 (31): 11089–96. doi:10.1021/bi980253t. PMID 9693005.
- Riento K, Galli T, Jansson S, Ehnholm C, Lehtonen E, Olkkonen VM (Sep 1998). "Interaction of Munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells". Journal of Cell Science. 111 (17): 2681–8. PMID 9701566.
- Guo Z, Turner C, Castle D (Aug 1998). "Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells". Cell. 94 (4): 537–48. doi:10.1016/S0092-8674(00)81594-9. PMID 9727496.
- Inoue T, Nielsen S, Mandon B, Terris J, Kishore BK, Knepper MA (Nov 1998). "SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles". The American Journal of Physiology. 275 (5 Pt 2): F752–60. doi:10.1152/ajprenal.1998.275.5.F752. PMID 9815132.
- Valdez AC, Cabaniols JP, Brown MJ, Roche PA (Mar 1999). "Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network". Journal of Cell Science. 112 (6): 845–54. PMID 10036234.
- Okamoto M, Schoch S, Südhof TC (Jun 1999). "EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis?". The Journal of Biological Chemistry. 274 (26): 18446–54. doi:10.1074/jbc.274.26.18446. PMID 10373452.
- Cabaniols JP, Ravichandran V, Roche PA (Dec 1999). "Phosphorylation of SNAP-23 by the novel kinase SNAK regulates t-SNARE complex assembly". Molecular Biology of the Cell. 10 (12): 4033–41. doi:10.1091/mbc.10.12.4033. PMC 25741. PMID 10588641.
- Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M (Mar 2000). "Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2". The Journal of Biological Chemistry. 275 (11): 8240–7. doi:10.1074/jbc.275.11.8240. PMID 10713150.
- Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M (Jun 2000). "Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment". Journal of Immunology. 164 (11): 5850–7. doi:10.4049/jimmunol.164.11.5850. PMID 10820264.
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH (May 2000). "SNAREs contribute to the specificity of membrane fusion". Neuron. 26 (2): 457–64. doi:10.1016/S0896-6273(00)81177-0. PMID 10839363.
- Martín-Martín B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F (Oct 2000). "Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis". Blood. 96 (7): 2574–83. PMID 11001914.
- Faigle W, Colucci-Guyon E, Louvard D, Amigorena S, Galli T (Oct 2000). "Vimentin filaments in fibroblasts are a reservoir for SNAP23, a component of the membrane fusion machinery". Molecular Biology of the Cell. 11 (10): 3485–94. doi:10.1091/mbc.11.10.3485. PMC 15008. PMID 11029050.
- Lazo PA, Nadal M, Ferrer M, Area E, Hernández-Torres J, Nabokina SM, Mollinedo F, Estivill X (Mar 2001). "Genomic organization, chromosomal localization, alternative splicing, and isoforms of the human synaptosome-associated protein-23 gene implicated in vesicle-membrane fusion processes". Human Genetics. 108 (3): 211–5. doi:10.1007/s004390100480. PMID 11354632.
- Shukla A, Corydon TJ, Nielsen S, Hoffmann HJ, Dahl R (Jul 2001). "Identification of three new splice variants of the SNARE protein SNAP-23". Biochemical and Biophysical Research Communications. 285 (2): 320–7. doi:10.1006/bbrc.2001.5144. PMID 11444845.
- Logan MR, Lacy P, Bablitz B, Moqbel R (Feb 2002). "Expression of eosinophil target SNAREs as potential cognate receptors for vesicle-associated membrane protein-2 in exocytosis". The Journal of Allergy and Clinical Immunology. 109 (2): 299–306. doi:10.1067/mai.2002.121453. PMID 11842301.