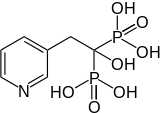
Risedronic acid
Risedronic acid, often used as its sodium salt risedronate sodium, is a bisphosphonate used to strengthen bone, treat or prevent osteoporosis, and treat Paget's disease of bone.
 | |
| Clinical data | |
|---|---|
| Trade names | Actonel, Atelvia, Benet, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.63% |
| Protein binding | ~24% |
| Metabolism | None |
| Elimination half-life | 1.5 h |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.116.436 |
| Chemical and physical data | |
| Formula | C7H11NO7P2 |
| Molar mass | 283.112 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It was patented in 1984 and approved for medical use in 1998.[1]
Pharmacology
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
Brand names
It is produced and marketed by Warner Chilcott, Sanofi-Aventis, and in Japan by Takeda under the trade names Actonel, Atelvia, and Benet. It is also available in a preparation that includes a calcium carbonate supplement, as Actonel with Calcium.
Controversies
In January 2006 P&G and its marketing partner Sanofi-Aventis filed a Lanham Act false claims lawsuit against rival drugmakers Roche and GlaxoSmithKline claiming false advertising about Boniva.[3] The manufacturers of Boniva, a rival bisphosphonate, were accused in the suit of causing a "serious public health risk" through misrepresentation of scientific findings. In a ruling on September 7, 2006 U.S. District Judge Paul A. Crotty rejected P&G's attempted injunction. P&G was criticized for attempting to "preserve its market share by denigrating Boniva". Judge Crotty wrote that "Roche was clearly entitled to respond with its own data, provided that the data was truthfully and accurately presented".[4]
In 2006, P&G faced controversy over its handling of clinical research involving risedronate (News Reports[5] and discussion).[6]
In common with other bisphosphonate drugs, risedronate appears to be associated with the rare side effect osteonecrosis of the jaw, often preceded by dental procedures inducing trauma to the bone.
See also
References
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 523. ISBN 9783527607495.
- D., Tripathi, K. (30 September 2013). Essentials of medical pharmacology (Seventh ed.). New Delhi. ISBN 9789350259375. OCLC 868299888.
- "P&G Press statement". Uk.pg.com. Retrieved 2013-03-01.
- NY fed judge finds promotions for bone drug Boniva are fair Associated Press, 7 Sept 2006
- http://www.thejabberwock.org/wiki/index.php?title=Actonel_Case_Media_Reports
- "Scientific Misconduct Blog". Scientific-misconduct.blogspot.com. Retrieved 2013-03-01.
External links
- "Risedronic acid". Drug Information Portal. U.S. National Library of Medicine.
- "Risedronate sodium". Drug Information Portal. U.S. National Library of Medicine.