Ring-opening metathesis polymerisation
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous and homogeneous catalysts have been developed. Most large-scale commercial processes rely on the former while some fine chemical syntheses rely on the homogeneous catalysts.[1] Catalysts are based on transition metals such as W, Mo, Re, Ru, and Ti.[2]
Heterogeneous catalysis and applications
 ROMP reaction giving polynorbornene. Like most commercial alkene metathesis processes, this reaction does not employ a well-defined molecular catalyst.
ROMP reaction giving polynorbornene. Like most commercial alkene metathesis processes, this reaction does not employ a well-defined molecular catalyst.
Ring-opening metathesis polymerization of cycloalkenes has been commercialized since the 1970s.[1] Examples of polymers produced on an industrial level through ROMP catalysis are Vestenamer or trans-polyoctenamer, which is the metathetical polymer of cyclooctene. Norsorex or polynorbornene is another important ROMP product on the market. Telene and Metton are polydicyclopentadiene products produced in a side reaction of the polymerization of norbornene.[3]
The ROMP process is useful because a regular polymer with a regular amount of double bonds is formed. The resulting product can be subjected to partial or total hydrogenation, or can be functionalized into more complex compounds.[3]
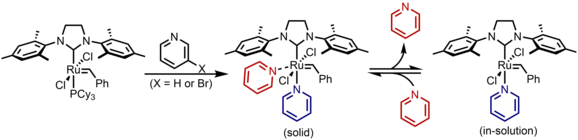
Homogeneous catalysts
The most common homogeneous catalyst for ROMP is also the best understood. In particular, the third generation Grubbs' catalyst(known as G3) has excellent functional group tolerance, air-stability, and fast initiation and propagation rates.[4][5][6] The fast initiation rates of G3 results in a living polymerization with narrow polymer molecular weight disperses. Which has made ROMP a popular choice for making advanced polymer architectures and functional polymer products.[7] It is common to quench these reactions with ethyl vinyl ether to generate the free polymer chain and an inactive Ru Fischer carbene complex.[8]
Mechanism
The mechanism of ROMP is similar to any olefin metathesis reaction. Initiation occurs by formation of an open coordination site. Propagation occurs via a metallacyclobutane intermediate.
Frontal ring-opening metathesis polymerization
Frontal ring-opening metathesis polymerization (FROMP) is a variation of ROMP in which it is a latent polymerization system that react fast, only upon ignition.[9] One example of this system is the FROMP of dicyclopentadiene with a Grubbs' catalyst initiated by heat.[10]
References
- Lionel Delaude, Alfred F. Noels (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 978-0471238966.CS1 maint: uses authors parameter (link)
- Grant), Cowie, J. M. G. (John McKenzie (2008). Polymers : chemistry and physics of modern materials. Arrighi, V. (Valeria) (3rd ed.). Boca Raton: CRC Press. ISBN 9780849398131. OCLC 82473191.
- Mol, J.C. (2004). "Industrial applications of olefin metathesis". Journal of Molecular Catalysis A: Chemical. 213 (1): 39–45. doi:10.1016/j.molcata.2003.10.049.
- Love, Jennifer A.; Morgan, John P.; Trnka, Tina M.; Grubbs, Robert H. (2002). "A Practical and Highly Active Ruthenium‐Based Catalyst that Effects the Cross Metathesis of Acrylonitrile". Angewandte Chemie International Edition. 41 (21): 4035–4037. doi:10.1002/1521-3773(20021104)41:21<4035::aid-anie4035>3.0.co;2-i. ISSN 1521-3773.
- Walsh, Dylan J.; Lau, Sii Hong; Hyatt, Michael G.; Guironnet, Damien (2017-09-25). "Kinetic Study of Living Ring-Opening Metathesis Polymerization with Third-Generation Grubbs Catalysts". Journal of the American Chemical Society. 139 (39): 13644–13647. doi:10.1021/jacs.7b08010. ISSN 0002-7863. PMID 28944665.
- Slugovc, Christian (2004-07-21). "The Ring Opening Metathesis Polymerisation Toolbox". Macromolecular Rapid Communications. 25 (14): 1283–1297. doi:10.1002/marc.200400150. ISSN 1022-1336.
- Sveinbjörnsson, Benjamin R.; Weitekamp, Raymond A.; Miyake, Garret M.; Xia, Yan; Atwater, Harry A.; Grubbs, Robert H. (2012-09-04). "Rapid self-assembly of brush block copolymers to photonic crystals". Proceedings of the National Academy of Sciences. 109 (36): 14332–14336. Bibcode:2012PNAS..10914332S. doi:10.1073/pnas.1213055109. PMC 3437898. PMID 22912408.
- Grubbs, R.H.; Tumas, W. (1989). "Polymer Synthesis and Organotransition Metal Chemistry". Science. 243 (4893): 907–915. Bibcode:1989Sci...243..907G. doi:10.1126/science.2645643. PMID 2645643.
- Ruiu, Andrea; Sanna, Davide; Alzari, Valeria; Nuvoli, Daniele; Mariani, Alberto (2014-07-15). "Advances in the frontal ring opening metathesis polymerization of dicyclopentadiene". Journal of Polymer Science Part A: Polymer Chemistry. 52 (19): 2776–2780. Bibcode:2014JPoSA..52.2776R. doi:10.1002/pola.27301. ISSN 0887-624X.
- Moneypenny, Timothy P.; Liu, Huiying; Yang, Anna; Robertson, Ian D.; Moore, Jeffrey S. (2017-04-13). "Grubbs-inspired metathesis in the Moore group". Journal of Polymer Science Part A: Polymer Chemistry. 55 (18): 2935–2948. Bibcode:2017JPoSA..55.2935M. doi:10.1002/pola.28592. ISSN 0887-624X.