Chain-growth polymerization
Chain growth polymerization (American spelling) or chain-growth polymerisation (English spelling) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time.[1] There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
Introduction
proceeds exclusively by reaction(s) between monomer(s) and active site(s)
on the polymer chain with regeneration of the active site(s) at the end of
each growth step.[2]
In 1953, Paul Flory first classified polymerization as “step-growth polymerization” and “chain-growth polymerization”.[3] IUPAC recommend to further simplified “chain-growth polymerization” to “chain polymerization”. It is a kind polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint.[4]
Many common polymers can be obtained by chain polymerization such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polymethyl methacrylate, polyacrylonitrile, polyvinyl acetate.[5]
Typically, chain-growth polymerization can be understood with the chemical equation:
In this equation, P is the polymer while x represents degree of polymerization, * means active center of chain-growth polymerization, M is the monomer which will react with active center, L is a low-molar-mass by-product obtained during chain propagation. Usually, for chain-growth polymerization, there is no by-product formed. However, there are still some exceptions. For example, amino acid N-carboxy anhydrides polymerizing to oxazolidine-2,5-diones.
Steps of chain-growth polymerization
Typically, chain polymerization must contain chain initiation and chain propagation. Chain transfer and chain termination do not always happen in a chain-growth polymerization.
Chain initiation
Chain initiation is the process of initially generating a chain carrier (chain carriers are some intermediates such as radical and ions in chain propagation process) in a chain polymerization. According to different ways of energy dissipation, it can be divided into thermal initiation, high energy initiation, and chemical initiation, etc. Thermal initiation is a process that obtained energy and dissociated to homolytic cleavage to form active center by molecular thermal motion. High energy initiation refers to the generation of chain carriers by radiation. Chemical initiation is due to chemical initiator.
Chain propagation
IUPAC defined chain propagation as an active center on the growing polymer molecule, which adds one monomer molecule to form a new polymer molecule which is one repeat unit longer with a new active center.
Chain transfer
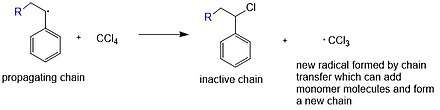
The polymerization process does not have to undergo chain transfer. Chain transfer means that in a chain polymerization, the active center of the polymer A takes an atom from B molecule and terminates. The B molecule produces a new active center instead. It can happen in free radical polymerization, ionic polymerization and coordination polymerization. Generally, chain transfer will generate by-product and in most of cases decreases molar mass of prepared polymer.[6]
Chain termination
Chain termination refers to in chain polymerization process, active center disappears, resulting in the termination of chain propagation. It is different from chain transfer. During the chain transfer process, the active point only shifts to another molecule but does not disappear.
Classes of chain-growth polymerization
Radical polymerization
Based on definition from IUPAC, radical polymerization is a chain polymerization in which the kinetic-chain carriers are radicals. Usually, the growing chain end bears an unpaired electron. Free radicals can be initiated by many methods such as heating, redox reactions, ultraviolet radiation, high energy irradiation, electrolysis, sonication, and plasma. Free radical polymerization is very important in polymer chemistry. It is one of the most developed method in chain-growth polymerization. Currently, most polymers in our daily life are synthesized by free radical polymerization, such as polyethylene, polystyrene, polyvinyl chloride, polymethyl methacrylate, polyacrylonitrile, polyvinyl acetate, styrene butadiene rubber, nitrile rubber, neoprene, etc.
Ionic polymerization
Based on IUPAC, ionic polymerization is a chain polymerization in which the kinetic-chain carriers are ions or ion pairs. It can be further divided into anionic polymerization and cationic polymerization. Ionic polymerization is widely used in our daily life. A lot of common polymers are generated by ionic polymerization such as butyl rubber, polyisobutylene, polyphenylene, polyoxymethylene, polysiloxane, polyethylene oxide, high density polyethylene, isotactic polypropylene, butadiene rubber, etc. Living anionic polymerization was developed since the 1950s, the chain will remain active indefinitely unless the reaction is transferred or terminated deliberately, which realizes the control of molar weight and PDI.[7]
Coordination polymerization
Based on definition from IUPAC, coordination polymerization is a chain polymerization that involves the preliminary coordination of a monomer molecule with a chain carrier. The monomer is firstly coordinated with the transition metal active center, and then the activated monomer is inserted into the transition metal-carbon bond for chain growth. In some cases, coordination polymerization is also called insertion polymerization or complexing polymerization. Advanced coordination polymerizations can control the tacticity, molecular weight and PDI of the polymer effectively. In addition, the racemic mixture of the chiral metallocene can be separated into its enantiomers. The oligomerization reaction produces an optically active branched olefin using an optically active catalyst.[8]
Living polymerization
Living polymerization was first introduced by Michael Szwarc in 1956. Based on definition from IUPAC, it is a chain polymerization from which chain transfer and chain termination are absent. As there is no chain-transfer and chain termination, the monomer in the system is consumed and the polymerization is stopped when the polymer chain remains active. Once the new monomer is added, the polymerization can proceed. Due to the low PDI and predictable molecular weight, living polymerization is at the forefront of polymer research. It could be further divided into living free radical polymerization, living ionic polymerization and living ring-opening metathesis polymerization, etc.
Ring-opening polymerization
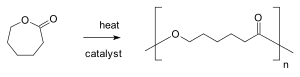
According to definition from IUPAC ring-opening polymerization is a polymerization in which a cyclic monomer yields a monomeric unit which is acyclic or contains fewer cycles than the monomer. Generally, the ring-opening polymerization is carried out under mild conditions, and the by-product is less than the polycondensation reaction and the high molecular weight polymer is easily obtained. Common ring-opening polymerization products includes polypropylene oxide, polytetrahydrofuran, polyepichlorohydrin, polyoxymethylene, polycaprolactam and polysiloxane.[9]
Reversible-deactivation polymerization
IUPAC stipulates that reversible-deactivation polymerization is a kind of chain polymerization, which is propagated by chain carriers that are deactivated reversibly, bringing them into active-dormant equilibria of which there might be more than one. An example of a reversible-deactivation polymerization is group-transfer polymerization.
Comparison to other polymerization methods
Previously, based on the difference between condensation reaction and addition reaction, Wallace Carothers classified polymerization as condensation polymerizations and addition polymerizations in 1929. However, Carothers’ classification is not good enough in mechanism aspect, as in some case, addition polymerizations shows condensation features while condensation polymerization shows addition features. Then the classification was optimized as step-growth polymerization and chain-growth polymerization. Based on IUPAC recommendation, the names of step-growth polymerization and chain-growth polymerization were further simplified as polyaddition and chain polymerization.
Step-growth polymerization
A step-growth reaction could happened between any two molecules with same or different degree of polymerization, usually monomers will form dimers, trimers, in matrix and finally react to long chain polymers. The mechanism of step-growth reaction is based on their functional group. Step-growth polymerization includes polycondensation and polyaddition. Polycondensation is a kind of polymerization whose chain growth is based on condensation reaction between two molecules with various degree of polymerization. The typical example are polyesters, polyamides and polyethers. It is sometimes confused by condensation previous definition of condensation polymerization. Polyaddition is a type of step-growth polymerization of which chain growth is based on addition reaction between two molecules of various degree of polymerization. The typical example for polyaddition is the synthesis of polyurethane. Compared to chain-growth polymerization, where the production of the growing chaingrowth is based on the reaction between polymer with active center and monomer, step-growth polymerization doesn’t have initiator or termination. The monomer in step-growth polymerization will be consumed very quickly to dimer, trimer or oligomer. The degree of polymerization will increase steady during the whole polymerization process. On the other hand, in chain-growth polymerization, the monomer consumed steadily but the degree of polymerization could be increased very quickly after chain initiation.[10] Compared to step-growth polymerization, living chain-growth polymerization shows low PDI, predictable molecular mass and controllable conformation. Some researchers are working on the transformation of two polymerization methods. Generally, polycondensation proceeds in a step-growth polymerization mode. Substituent effect, catalyst transfer and biphasic system could be used for inhibiting the activity of monomer, and further prevent monomers from reacting with each other. It could make polycondensation process go in a chain-growth polymerization mode.
Polycondensation
The chain growth of polycondensation is based on condensation reaction. Low-molar-mass by-product will be formed during polymerization. It is a previous way to classify polymerization, which was introduced by Carothers in 1929. It is still used currently in some case. The step-growth polymerization with low-molar-mass by-product during chain growth is defined as polycondensation. The chain-growth polymerization a with low-malar-mass by-product during chain growth is recommended by IUPAC as “condensative chain polymerization”.[11]
Addition polymerization
Addition polymerization is also a type of previous definition. The chain growth of addition polymerization is based addition reactions. There is no low-molar-mass by-product formed during polymerization. The step-growth polymerization based on addition reaction during chain growth is defined as polyaddition. Based on that definition, the addition polymerization contains both polyaddition and chain polymerization except condensative chain polymerization we used now.
Application
Chain polymerization products are widely used in many aspects of life, including electronic devices, food packaging, catalyst carriers, medical materials, etc. At present, the world's highest yielding polymers such as polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), etc. can be obtained by chain polymerization. In addition, some carbon nanotube polymer is used for electronical devices. Controlled living chain-growth conjugated polymerization will also enable the synthesis of well-defined advanced structures, including block copolymers. Their industrial applications extend to water purification, biomedical devices and sensors.[12]
References
- Introduction to Polymers 1987 R.J. Young Chapman & Hall ISBN 0-412-22170-5
- Penczek, Stanisław; Moad, Graeme (2008). "Glossary of terms related to kinetics, thermodynamics, and mechanisms of polymerization (IUPAC Recommendations 2008)" (PDF). Pure and Applied Chemistry. 80 (10): 2163–2193. doi:10.1351/pac200880102163.
- R.J.Young (1983). Introduction to polymers. Chapman and Hall. ISBN 0-412-22170-5.
- Plastics packaging : Properties, processing, applications, and regulations (2nd ed.). Hanser Pub. 2004. ISBN 1-56990-372-7.
- Paul Flory (1953). Principles of polymer chemistry. Cornell University Press. ISBN 0-8014-0134-8.
- Paul Flory (1953). Principles of polymer chemistry. Cornell University Press. ISBN 0-8014-0134-8.
- Sawamoto, Mitsuo (January 1991). "Modern cationic vinyl polymerization". Progress in Polymer Science. 16 (1): 111–172. doi:10.1016/0079-6700(91)90008-9.
- Kaminsky, Walter (1 January 1998). "Highly active metallocene catalysts for olefin polymerization". Journal of the Chemical Society, Dalton Transactions (9): 1413–1418. doi:10.1039/A800056E. ISSN 1364-5447.
- Hofsten, E. "Population growth-a menace to what?". Polymer Journal. ISSN 1349-0540.
- Aplan, Melissa P.; Gomez, Enrique D. (3 July 2017). "Recent Developments in Chain-Growth Polymerizations of Conjugated Polymers". Industrial & Engineering Chemistry Research. 56 (28): 7888–7901. doi:10.1021/acs.iecr.7b01030.
- Herzog, Ben; Kohan, Melvin I.; Mestemacher, Steve A.; Pagilagan, Rolando U.; Redmond, Kate (2013). "Polyamides". Ullmann's Encyclopedia of Industrial Chemistry. American Cancer Society. doi:10.1002/14356007.a21_179.pub3. ISBN 978-3527306732.
- Sawamoto, Mitsuo (January 1991). "Modern cationic vinyl polymerization". Progress in Polymer Science. 16 (1): 111–172. doi:10.1016/0079-6700(91)90008-9.