Retinoschisin
Retinoschisin also known as X-linked juvenile retinoschisis protein is a lectin[5][6] that in humans is encoded by the RS1 gene.[7]
It is a soluble, cell-surface protein that plays an important role in the maintenance of the retina where it is expressed and secreted by retinal bipolar cells and photoreceptors,[8][9] as well as in the pineal gland.[10] Retinoschisin (RS1) is encoded by the gene RS1 located on the X chromosome at p22.1.[7] Young males who have an RS1 mutation are susceptible to retinoschisis, and X-linked eye disease which causes macular degeneration and can lead to a loss of vision.[5][9]
Function
Retinoschisin is an extracellular protein that plays a crucial role in the cellular organization of the retina: it binds the plasma membranes of various retinal cells tightly to maintain the structure of the retina.[5] In addition to enabling cell-to-cell adhesion, it has been shown that retinoschisin interacts with the sodium/potassium-ATPase (Na/K-ATPase) which resides in the plasma membrane.[10] RS1 also plays a role in the regulation on intracellular MAP kinase signalling.[11]
Structure
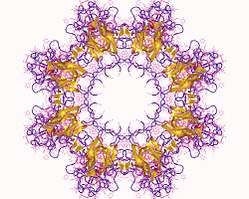
The retinoschisin monomer is 224 amino acids long,[7] including a 23-amino acid signal peptide essential for secretion[5] (this is cleaved off before the protein becomes functional), and a highly conserved sequence motif called the discoidin domain which consists of 157 amino acids,[12] important for the protein's function in cell to cell adhesion.[13] However, its oligomeric structure is a pairing of back-to-back octamers,[8] forming a homo16mer . This structure allows it to adhere to the plasma membrane of retinal cells such as bipolar and photoreceptor cells,[9] joining them together.
Clinical significance
Pathogenic mutations of this gene are responsible for X-linked retinoschisis an early-onset macular degeneration in males that results in a splitting of the inner layers of the retina and severe loss in vision.[14] Female carriers of the RS1 mutation do not show symptoms of X-linked juvenile retinoschisis, except in rare cases where the non-functional protein is expressed due to anomalous X-chromosome inactivation. In young males who carry a gene mutation, the disease presents itself as retinal cavities, splitting of inner retinal layers (also known as foveal schisis),[8][5] and defective synapse activity.[5][12] Retinas that lack mature retinoshisin develop these characteristics in up to 1 in 5,000 males.[11] There are over 200 mutations of RS1 recorded in the Retina International Mutation Database, most of which are not pathogenic.
References
- GRCh38: Ensembl release 89: ENSG00000102104 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000031293 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Vijayasarathy C, Ziccardi L, Sieving PA (2012). "Biology of retinoschisin". Advances in Experimental Medicine and Biology. 723: 513–8. doi:10.1007/978-1-4614-0631-0_64. ISBN 978-1-4614-0630-3. PMC 3475158. PMID 22183371.
- Wu WW (October 2005). RS1 structure-function relationships: roles in retinal adhesion and X-linked retinoschisis (Ph.D. thesis). The University of British Columbia.
- Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, Lorenz B, Jurklies B, Weber BH (October 1997). "Positional cloning of the gene associated with X-linked juvenile retinoschisis". Nature Genetics. 17 (2): 164–70. doi:10.1038/ng1097-164. PMID 9326935.
- Tolun G, Vijayasarathy C, Huang R, Zeng Y, Li Y, Steven AC, Sieving PA, Heymann JB (May 2016). "Paired octamer rings of retinoschisin suggest a junctional model for cell-cell adhesion in the retina". Proceedings of the National Academy of Sciences of the United States of America. 113 (19): 5287–92. doi:10.1073/pnas.1519048113. PMC 4868477. PMID 27114531.
- Kotova S, Vijayasarathy C, Dimitriadis EK, Ikonomou L, Jaffe H, Sieving PA (August 2010). "Retinoschisin (RS1) interacts with negatively charged lipid bilayers in the presence of Ca2+: an atomic force microscopy study". Biochemistry. 49 (33): 7023–32. doi:10.1021/bi1007029. PMC 2929131. PMID 20677810.
- Plössl K, Royer M, Bernklau S, Tavraz NN, Friedrich T, Wild J, Weber BH, Friedrich U (August 2017). "Retinoschisin is linked to retinal Na/K-ATPase signaling and localization". Molecular Biology of the Cell. 28 (16): 2178–2189. doi:10.1091/mbc.e17-01-0064. PMC 5531734. PMID 28615319.
- Plössl K, Schmid V, Straub K, Schmid C, Ammon M, Merkl R, Weber BH, Friedrich U (July 2018). "Pathomechanism of mutated and secreted retinoschisin in X-linked juvenile retinoschisis". Experimental Eye Research. 177: 23–34. doi:10.1016/j.exer.2018.07.021. PMID 30040949.
- Reid SN, Yamashita C, Farber DB (July 2003). "Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells". The Journal of Neuroscience. 23 (14): 6030–40. doi:10.1523/JNEUROSCI.23-14-06030.2003. PMC 6740352. PMID 12853421.
- Wu WW, Molday RS (July 2003). "Defective discoidin domain structure, subunit assembly, and endoplasmic reticulum processing of retinoschisin are primary mechanisms responsible for X-linked retinoschisis". The Journal of Biological Chemistry. 278 (30): 28139–46. doi:10.1074/jbc.M302464200. PMID 12746437.
- Weber BH, Kellner U (2007). "X-Linked Juvenile Retinoschisis". In Tombran-Tink J, Barnstable C (eds.). Retinal Degenerations: Biology, Diagnostics, and Therapeutics. Springer Science & Business Media. pp. 119–135. ISBN 978-1-59745-186-4.
Further reading
- Sikkink SK, Biswas S, Parry NR, Stanga PE, Trump D (April 2007). "X-linked retinoschisis: an update". Journal of Medical Genetics. 44 (4): 225–32. doi:10.1136/jmg.2006.047340. PMC 2598044. PMID 17172462.
- Alitalo T, Kruse TA, de la Chapelle A (March 1991). "Refined localization of the gene causing X-linked juvenile retinoschisis". Genomics. 9 (3): 505–10. doi:10.1016/0888-7543(91)90417-D. PMID 2032721.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Hotta Y, Fujiki K, Hayakawa M, Ohta T, Fujimaki T, Tamaki K, Yokoyama T, Kanai A, Hirakata A, Hida T, Nishina S, Azuma N (August 1998). "Japanese juvenile retinoschisis is caused by mutations of the XLRS1 gene". Human Genetics. 103 (2): 142–4. doi:10.1007/pl00008705. PMID 9760195.
- Shastry BS, Hejtmancik FJ, Trese MT (March 1999). "Recurrent missense (R197C) and nonsense (Y89X) mutations in the XLRS1 gene in families with X-linked retinoschisis". Biochemical and Biophysical Research Communications. 256 (2): 317–9. doi:10.1006/bbrc.1999.0323. PMID 10079181.
- Mashima Y, Shinoda K, Ishida S, Ozawa Y, Kudoh J, Iwata T, Oguchi Y, Shimizu N (1999). "Identification of four novel mutations of the XLRS1 gene in Japanese patients with X-linked juvenile retinoschisis. Mutation in brief no. 234. Online". Human Mutation. 13 (4): 338. doi:10.1002/(SICI)1098-1004(1999)13:4<338::AID-HUMU16>3.0.CO;2-0. PMID 10220153.
- Huopaniemi L, Rantala A, Forsius H, Somer M, de la Chapelle A, Alitalo T (April 1999). "Three widespread founder mutations contribute to high incidence of X-linked juvenile retinoschisis in Finland". European Journal of Human Genetics. 7 (3): 368–76. doi:10.1038/sj.ejhg.5200300. PMID 10234514.
- Gehrig A, White K, Lorenz B, Andrassi M, Clemens S, Weber BH (June 1999). "Assessment of RS1 in X-linked juvenile retinoschisis and sporadic senile retinoschisis". Clinical Genetics. 55 (6): 461–5. doi:10.1034/j.1399-0004.1999.550611.x. PMID 10450864.
- Hiriyanna KT, Bingham EL, Yashar BM, Ayyagari R, Fishman G, Small KW, Weinberg DV, Weleber RG, Lewis RA, Andreasson S, Richards JE, Sieving PA (2000). "Novel mutations in XLRS1 causing retinoschisis, including first evidence of putative leader sequence change". Human Mutation. 14 (5): 423–7. doi:10.1002/(SICI)1098-1004(199911)14:5<423::AID-HUMU8>3.0.CO;2-D. hdl:2027.42/35178. PMID 10533068.
- Grayson C, Reid SN, Ellis JA, Rutherford A, Sowden JC, Yates JR, Farber DB, Trump D (July 2000). "Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells". Human Molecular Genetics. 9 (12): 1873–9. doi:10.1093/hmg/9.12.1873. PMID 10915776.
- Weber BH, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, Jaissle GB, Friedburg C, Tamm E, Molday RS (April 2002). "Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure". Proceedings of the National Academy of Sciences of the United States of America. 99 (9): 6222–7. doi:10.1073/pnas.092528599. PMC 122930. PMID 11983912.
- Tuvdendorj D, Isashiki Y, Ohba N, Sonoda S, Izumo S (June 2002). "Two Japanese patients with mutations in the XLRS1 gene". Retina. 22 (3): 354–7. doi:10.1097/00006982-200206000-00017. PMID 12055472.
- Wistow G, Bernstein SL, Wyatt MK, Ray S, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K (June 2002). "Expressed sequence tag analysis of human retina for the NEIBank Project: retbindin, an abundant, novel retinal cDNA and alternative splicing of other retina-preferred gene transcripts". Molecular Vision. 8: 196–204. PMID 12107411.
- Inoue Y, Yamamoto S, Inoue T, Fujikado T, Kusaka S, Ohguro N, Ohji M, Tano Y (October 2002). "Two novel point mutations of the XLRS1 gene in patients with X-linked juvenile retinoschisis". American Journal of Ophthalmology. 134 (4): 622–4. doi:10.1016/S0002-9394(02)01592-1. PMID 12383832.
- Wang T, Waters CT, Rothman AM, Jakins TJ, Römisch K, Trump D (November 2002). "Intracellular retention of mutant retinoschisin is the pathological mechanism underlying X-linked retinoschisis". Human Molecular Genetics. 11 (24): 3097–105. doi:10.1093/hmg/11.24.3097. PMID 12417531.
- Tanimoto N, Usui T, Takagi M, Hasegawa S, Abe H, Sekiya K, Miyagawa Y, Nakazawa M (2003). "Electroretinographic findings in three family members with X-linked juvenile retinoschisis associated with a novel Pro192Thr mutation of the XLRS1 gene". Japanese Journal of Ophthalmology. 46 (5): 568–76. doi:10.1016/S0021-5155(02)00539-7. PMID 12457918.
- Fraternali F, Cavallo L, Musco G (June 2003). "Effects of pathological mutations on the stability of a conserved amino acid triad in retinoschisin". FEBS Letters. 544 (1–3): 21–6. doi:10.1016/S0014-5793(03)00433-2. PMID 12782284.
- Sato M, Oshika T, Kaji Y, Nose H (2003). "Three novel mutations in the X-linked juvenile retinoschisis (XLRS1) gene in 6 Japanese patients, 1 of whom had Turner's syndrome". Ophthalmic Research. 35 (5): 295–300. doi:10.1159/000072151. PMID 12920343.
External links
This article incorporates text from the United States National Library of Medicine, which is in the public domain.