Reducing equivalent
In biochemistry, the term reducing equivalent refers to any of a number of chemical species which transfer the equivalent of one electron in redox reactions.[1] A redox reaction (oxidation-reduction reaction) results in a change of oxidation state of atoms or ions due to actual or formal transfer of electrons. A chemical species is reduced when it gains electrons and is oxidized when it loses electrons.[2] A reducing equivalent serves as the electron donor in a redox reaction and becomes oxidized (loses electrons) when it donates an electron to an electron acceptor.[3] A reducing equivalent can donate an electron in multiple ways: as a lone electron, as a hydrogen atom, as a hydride ion, or by bond formation with an oxygen atom.[4]
Lone electrons
In redox reactions involving metal ions, lone electrons can be transferred from the electron donor to the electron acceptor. That is, no other atoms or protons are transferred along with the electron in the redox reaction. [5] For instance, consider the following reaction between iron and copper ions:

The iron and copper ion each begin the reaction with a double positive charge (2+). At the end of the reaction, the charge on copper decreased to a single positive charge (1+) while the charge on iron increased to a triple positive charge (3+). The alteration of charges is due to the transfer of a single electron from the iron atom to the copper atom.[5] In this reaction, iron is acting as the reducing equivalent because it donates one of its electrons to copper. As a result, iron is oxidized because it loses one of its electrons resulting in a greater positive charge.[5]
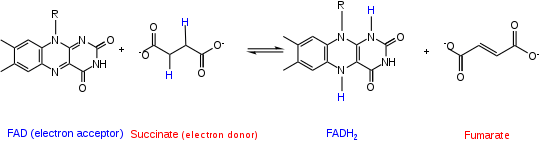
Hydrogen atom
A neutral hydrogen atom consists of one electron and one proton. Hydrogen's electronegativity value of 2.2 is less than the electronegativity of the atoms that hydrogen is commonly bound to, such as oxygen, nitrogen, carbon, or fluorine.[6] When a hydrogen atom forms a covalent bond with a more electronegative atom, the more electronegative atom will have a greater affinity for the electron and pull the electron partially away from hydrogen.[6] The more electronegative atom is reduced because it formally gains the electrons involved in the covalent bond. Conversely, when an atom loses a bond to a hydrogen atom it is oxidized because it loses electrons.[4] For example, consider the reaction involving Flavin Adenine Dinucleotide (FAD) and succinate. In this reaction FAD is reduced to FADH2 because it accepts two hydrogen atoms from succinate. Succinate serves as the reducing equivalent because it donates electrons to FAD in the form of hydrogen atoms and is itself oxidized.[7]

Hydride
A hydride ion is a hydrogen anion consisting of one proton and two electrons.[8] A chemical species that donates a hydride is a reducing equivalent because it donates the equivalence of at least one electron, regardless if it is in the form of a hydride.[4] The chemical species that accepts a hydride ion will be reduced because it gains the electrons from the hydride ion.[4] The reduced form of Nicotinamide Adenine Dinucleotide (NADH) is a reducing equivalent commonly seen in biochemistry that donates a hydride ion to an electron acceptor in complex I of the mitochondrial electron transport chain.[3]
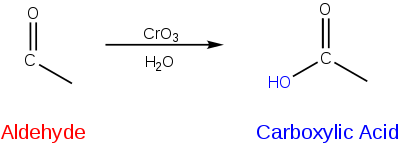
Bond formation with oxygen atom
A chemical species with a lower electronegativity than oxygen can serve as a reducing equivalent when it covalently binds to an oxygen atom. Oxygen is highly electronegative and will have a greater affinity for electrons in a covalent bond, resulting in the reduction of the oxygen atom.[4] When an atom of lower electronegativity forms a bond with oxygen it is oxidized because the electrons are pulled closer to oxygen and away from that atom.[6][4] For instance, consider the formation of a carboxylic acid from the oxidation of an aldehyde. In this reaction, a carbon has been oxidized through the formation of a covalent bond with oxygen.[4]
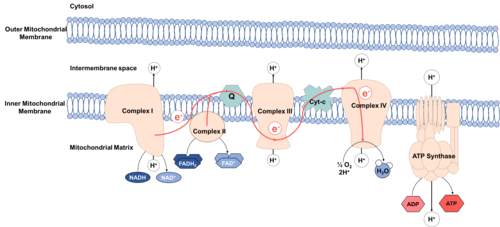
Reducing equivalents in the mitochondrial respiratory chain
Oxidative phosphorylation is the process driving ATP synthesis by means of the energy of O2 [9] released through the oxidation of reducing equivalents. It occurs in the mitochondrial electron transport chain,[10][4] which is located at the inner mitochondrial membrane and consists of transmembrane protein complexes that catalyze redox reactions.[11] NADH and FADH2 are reducing equivalents that donate electrons at complexes I and II, respectively. These electrons are then transferred in multiple redox reactions and are carried to the complexes III and IV.[3][12] The oxidation of reducing equivalents in the electron transport chain releases protons into the intermembrane space of the mitochondria and maintains the proton electrochemical gradient.[3][4] As protons move down the electrochemical gradient through the transmembrane protein ATP synthase, ATP is generated from ADP and an inorganic phosphate group.[3][4] In order to maintain the proton gradient and generate ATP, reducing equivalents are supplied to the electron transport chain from multiple processes such as the TCA cycle.[13]
See also
References
- Jain JL (2004). Fundamentals of Biochemistry. S. Chand. ISBN 81-219-2453-7.
- Beiras R (2018-07-19). Marine pollution : sources, fate and effects of pollutants in coastal ecosystems. Amsterdam, Netherlands. ISBN 9780128137376. OCLC 1045426277.
- Voet D, Voet JG, Pratt CW (2013). Fundamentals of Biochemistry: Life at the Molecular Level (Fourth ed.). Hoboken, NJ. ISBN 9780470547847. OCLC 738349533.
- Lehninger AL, Nelson DL, Cox MM (2017-01-01). Lehninger principles of biochemistry (Seventh ed.). New York, NY. ISBN 9781464126116. OCLC 986827885.
- Damn HC, Goldwyn AJ, Thomas CA (1966). The handbook of biochemistry and biophysics. Cleveland, Ohio: The World Publishing Company. pp. 400–415.
- Atkins PW, Jones L, Laverman L. Chemical principles : the quest for insight (6th ed.). New York. ISBN 978-1429288972. OCLC 819816793.
- Stryer L, Tymoczko JL, Berg JM (2002). "The Citric Acid Cycle Oxidizes Two-Carbon Units". Biochemistry. 5th Edition: Section 17.1.8.
- Stenesh J (1975). Dictionary of biochemistry. New York: Wiley. ISBN 0471821055. OCLC 1530913.
- Schmidt-Rohr, K. (2020). "Oxygen Is the High-Energy Molecule Powering Complex Multicellular Life: Fundamental Corrections to Traditional Bioenergetics” ACS Omega 5: 2221-2233. http://dx.doi.org/10.1021/acsomega.9b03352
- Kehrer JP, Lund LG (July 1994). "Cellular reducing equivalents and oxidative stress". Free Radical Biology & Medicine. 17 (1): 65–75. doi:10.1016/0891-5849(94)90008-6. PMID 7959167.
- Begley TP (2009). Wiley encyclopedia of chemical biology. Hoboken, N.J.: John Wiley & Sons. ISBN 9780471754770. OCLC 243818536.
- Ahern K, Rajagopal I, Tan T (2018). Biochemistry Free for All. Creative Commons. p. 432.
- Montano-Herrera L, Laycock B, Werker A, Pratt S (March 2017). "The Evolution of Polymer Composition during PHA Accumulation: The Significance of Reducing Equivalents". Bioengineering. 4 (1): 20. doi:10.3390/bioengineering4010020. PMC 5590436. PMID 28952499.