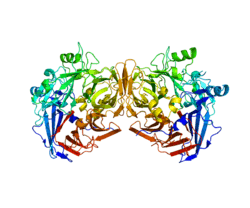RPE65
Retinal pigment epithelium-specific 65 kDa protein, also known as retinoid isomerohydrolase, is an enzyme of the vertebrate visual cycle that is encoded in humans by the RPE65 gene.[5][6] RPE65 is expressed in the retinal pigment epithelium (RPE, a layer of epithelial cells that nourish the photoreceptor cells) and is responsible for the conversion of all-trans-retinyl esters to 11-cis-retinol during phototransduction.[7] 11-cis-retinol is then used in visual pigment regeneration in photoreceptor cells.[8][9] RPE65 belongs to the carotenoid oxygenase family of enzymes.[8]
Function
RPE65 is a critical enzyme in the vertebrate visual cycle found the retinal pigmented epithelium. It is also found in rods and cones.[10] The photoisomerization of 11-cis-retinal to all-trans-retinal initiates the phototransduction pathway through which the brain detects light. All-trans-retinol is not photoactive and therefore must be reconverted to 11-cis-retinal before it can recombine with opsin to form an active visual pigment.[8][11] RPE65 reverses the photoisomerization by converting an all-trans-retinyl ester to 11-cis-retinol. Most commonly, the ester substrate is retinyl palmitate. The other enzymes of the visual cycle complete the reactions necessary to oxidize and esterify all-trans-retinol to a retinyl ester (RPE65's substrate) and to oxidize 11-cis-retinol to 11-cis-retinal (the required photoactive visual pigment component).[8][9]
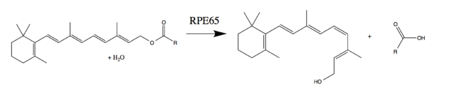
RPE65 is also referred to as retinol isomerase or retinoid isomerase, owing to past debates about the enzyme's substrate and whether it was involved in ester hydrolysis.[9]
Structure
RPE65 is a dimer of two symmetrical, enzymatically independent subunits. The active site of each subunit has a seven-bladed beta-propeller structure with four histidines that hold an iron(II) cofactor.[9][12] This structural motif is common across the studied members of the carotenoid oxygenase family of enzymes. RPE65 is strongly associated with the membrane of the smooth endoplasmic reticulum in RPE cells.[8]
Active site structure
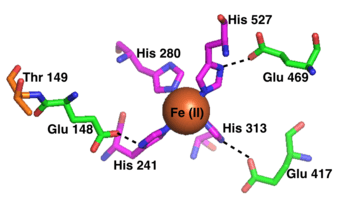
The active site of each RPE65 active site contains an Fe(II) cofactor bound by four histidines (His180, His241, His313, and His527), each contributed by a separate blade on the beta-propeller structure. Three of the four histidines are coordinated to nearby glutamic acid residues (Glu148, Glu417, and Glu469), which are thought to help position the histidines to bind the iron cofactor in an octahedral geometry.[13] Phe103, Thr147, and Glu148 surround the active site where they help stabilize the carbocation intermediate and increase the stereoselectivity of RPE65 for 11-cis-retinol over 13-cis-retinol.[9]
Reactants and products likely enter and leave the active site through a hydrophobic tunnel which is thought to open into the lipid membrane for direct lipid substrate absorption. A second, smaller tunnel also reaches the active site and may serve as a pathway for water, but is too narrow to transport the retinoid reactants and products.[9][13]
Membrane interactions
RPE65 is strongly associated with the membrane of the sER. sER is abnormally abundant in RPE cells due to their role in processing lipidic retinoids. Structural studies indicate that RPE65 is partially imbedded in the sER membrane via interactions between its hydrophobic face and the interior of the lipid membrane. This is supported by the need for detergent to solubilize RPE65. A major portion of RPE65’s hydrophobic face, residues 109-126, forms an amphipathic alpha helix that likely contributes to the protein’s membrane affinity. Additionally, Cys112 is palmitoylated in native RPE65, further supporting the theory that the hydrophobic face of RPE65 is imbedded in the membrane.[13]
The hydrophobic face contains the entrance to the large tunnel that leads to the enzyme’s active site. The presence of this channel on the hydrophobic face combined with RPE65’s demonstrated ability to absorb substrate direction from the lipid bilayer is consistent with RPE65 being partially embedded in the membrane.[8]
Conservation
RPE65 has been isolated from a wide range of vertebrates including zebra fish, chicken, mice, frogs, and humans.[8][14][15] Its structure is highly conserved between species, particularly in the beta-propeller and likely membrane bound regions. The amino acid sequences of human and bovine RPE65 differ by less than 1%.[13] The histidine residues of the beta-propeller structure and the bound iron(II) cofactor are 100% conserved across studied RPE65 orthologs and other members of the carotenoid oxygenase family.[9]
Soluble RPE65 (sRPE65)
Previously, it was proposed that RPE65 exists in two, interconverted forms: membrane bound mRPE65 and soluble sRPE65. This theory suggested that the reversible conversion of sRPE65 to mRPE65 by palmitoylation at Cys231, Cys329, and Cys330 played a role in regulating the retinoid cycle and endowing mRPE65 with its membrane affinity.[16] However, crystallographic studies of RPE65 have demonstrated that these residues are neither palmitoylated nor surface facing. New studies have also failed to confirm the presence of abundant soluble RPE65. Thus, this theory has been largely abandoned.[8][13]
Mechanism
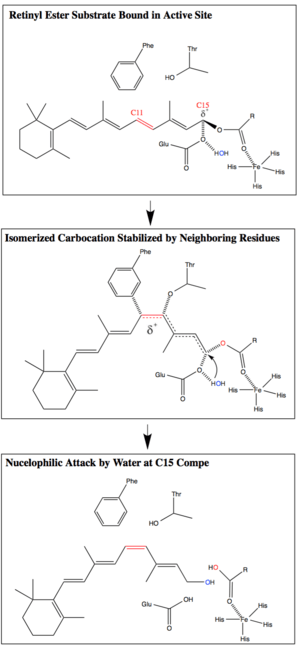
RPE65 catalyzes the conversion of all-trans-retinyl ester to 11-cis-retinol through a proposed SN1 O-alkyl bond cleavage. RPE65’s combination of an O-alkyl ester cleavage, geometric isomerization, and water addition is currently thought to be unique in biology. However, O-alkyl ester cleavage reactions with similarly stabilized carbocation intermediates are used by organic chemists.[9][17]
O-Alkyl cleavage
The O-alkyl cleavage of the ester bond, assisted by an Fe(II) cofactor, creates a carbocation intermediate that is stabilized by the conjugated polyene chain. The delocalization of the carbocation reduces the bond order of the polyene chain, thereby reducing the activation energy of the trans-to-cis isomerization. Phe103 and Thr178 additionally stabilize the isomerized carbocation and are thought to be responsible for the stereoselectivity of the enzyme. After isomerization, a nucleophilic attack by water at C15 restores the conjugation of the polyene chain and completes the ester bond cleavage.[9][13]
Alternate SN2 mechanism
Nearly all other biochemical ester hydrolysis reactions occur through SN2 reaction at the acyl carbon. However, isotope labeling studies have demonstrated that the oxygen on the final 11-cis-retinol product of RPE65 originates from the solvent rather than the reacting ester, supporting the O-alkyl cleavage mechanism.[13] Additionally, an SN2 ester hydrolysis reaction mechanism would rely on a separate, unfavorable SN2 attack at electron rich C11 by some nucleophile - most likely a cystine residue - to complete the isomerization portion of the reaction. Not only is nucleophilic attack of an alkene energetically unfavorable, but the active site region is lacking cystine residues to act as the nucleophile.[8][9]
Clinical significance
Mutations in this gene have been associated with Leber's congenital amaurosis type 2 (LCA2) and retinitis pigmentosa (RP).[6][18] RPE65 mutations are the most commonly detected mutations in LCA patients in Denmark.[19] The vast majority of RPE65 mutations in patients with LCA2 and RP occur in the beta-propeller regime and are believed to inhibit proper protein folding and iron cofactor binding. Particularly common propeller mutation sites are Tyr368 and His182. Substitution at Arg91 is also common and have been shown to impact RPE65 membrane interactions and substrate uptake.[13]
Though complete loss of function is associated with diseases such as LCA and RP, partial inhibition of RPE65 has been proposed as a treatment for age-related macular degeneration (AMD). All-trans-retinylamine (Ret-NH2) and emixustat have both been shown to competitively inhibit RPE65.[9] Emixustat is currently undergoing FDA phase 3 clinical trials as a therapy for AMD.[9][20]
Jean Bennett and Katherine A. High's work with the RPE65 mutation has reversed an inherited form of blindness. They received the first FDA approval of a gene therapy for a genetic disease. For this, in 2018, they were named as one of three finalists for Sanford Health's $1 million Lorraine Cross Award for innovation in science and medicine.
See also
- The Visual Cycle
References
- GRCh38: Ensembl release 89: ENSG00000116745 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028174 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM (Jul 1993). "Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro". The Journal of Biological Chemistry. 268 (21): 15751–7. PMID 8340400.
- "Entrez Gene: RPE65 retinal pigment epithelium-specific protein 65kDa".
- Wolf G (Mar 2005). "Function of the protein RPE65 in the visual cycle". Nutrition Reviews. 63 (3): 97–100. doi:10.1111/j.1753-4887.2005.tb00127.x. PMID 15825812.
- Kiser PD, Palczewski K (Sep 2010). "Membrane-binding and enzymatic properties of RPE65". Progress in Retinal and Eye Research. 29 (5): 428–42. doi:10.1016/j.preteyeres.2010.03.002. PMC 2903629. PMID 20304090.
- Kiser PD, Zhang J, Badiee M, Li Q, Shi W, Sui X, Golczak M, Tochtrop GP, Palczewski K (Jun 2015). "Catalytic mechanism of a retinoid isomerase essential for vertebrate vision". Nature Chemical Biology. 11 (6): 409–15. doi:10.1038/nchembio.1799. PMC 4433804. PMID 25894083.
- Tang, Peter H.; Buhusi, Mona C.; Ma, Jian-Xing; Crouch, Rosalie K. (2011-12-14). "RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model". The Journal of Neuroscience. 31 (50): 18618–18626. doi:10.1523/JNEUROSCI.4265-11.2011. ISSN 1529-2401. PMC 3297673. PMID 22171060.
- Kiser PD, Golczak M, Palczewski K (Jan 2014). "Chemistry of the retinoid (visual) cycle". Chemical Reviews. 114 (1): 194–232. doi:10.1021/cr400107q. PMC 3858459. PMID 23905688.
- Orban T, Jastrzebska B, Palczewski K (Apr 2014). "Structural approaches to understanding retinal proteins needed for vision". Current Opinion in Cell Biology. 27: 32–43. doi:10.1016/j.ceb.2013.11.001. PMC 3971393. PMID 24680428.
- Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K (Oct 2009). "Crystal structure of native RPE65, the retinoid isomerase of the visual cycle". Proceedings of the National Academy of Sciences of the United States of America. 106 (41): 17325–30. doi:10.1073/pnas.0906600106. PMC 2765077. PMID 19805034.
- Takahashi, Yusuke; Moiseyev, Gennadiy; Ma, Jian-xing (2014-09-26). "Identification of key residues determining isomerohydrolase activity of human RPE65". The Journal of Biological Chemistry. 289 (39): 26743–26751. doi:10.1074/jbc.M114.558619. ISSN 1083-351X. PMC 4175317. PMID 25112876.
- Jin M, Li S, Moghrabi WN, Sun H, Travis GH (Aug 2005). "Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium". Cell. 122 (3): 449–59. doi:10.1016/j.cell.2005.06.042. PMC 2748856. PMID 16096063.
- Ma J, Zhang J, Othersen KL, Moiseyev G, Ablonczy Z, Redmond TM, Chen Y, Crouch RK (Jun 2001). "Expression, purification, and MALDI analysis of RPE65". Investigative Ophthalmology & Visual Science. 42 (7): 1429–35. PMID 11381042.
- Redmond, T. Michael; Poliakov, Eugenia; Kuo, Stephanie; Chander, Preethi; Gentleman, Susan (2010-01-15). "RPE65, visual cycle retinol isomerase, is not inherently 11-cis-specific: support for a carbocation mechanism of retinol isomerization". The Journal of Biological Chemistry. 285 (3): 1919–1927. doi:10.1074/jbc.M109.027458. ISSN 1083-351X. PMC 2804350. PMID 19920137.
- Bowne SJ, Humphries MM, Sullivan LS, Kenna PF, Tam LC, Kiang AS, Campbell M, Weinstock GM, Koboldt DC, Ding L, Fulton RS, Sodergren EJ, Allman D, Millington-Ward S, Palfi A, McKee A, Blanton SH, Slifer S, Konidari I, Farrar GJ, Daiger SP, Humphries P (Oct 2011). "A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement". European Journal of Human Genetics. 19 (10): 1074–81. doi:10.1038/ejhg.2011.86. PMC 3190249. PMID 21654732.
- Astuti GD, Bertelsen M, Preising MN, Ajmal M, Lorenz B, Faradz SM, Qamar R, Collin RW, Rosenberg T, Cremers FP (Dec 2015). "Comprehensive genotyping reveals RPE65 as the most frequently mutated gene in Leber congenital amaurosis in Denmark". European Journal of Human Genetics. doi:10.1038/ejhg.2015.241. PMC 5070892. PMID 26626312.
- "Acucela - Retinal Diseases". acucela.com. Retrieved 2016-03-01.
Further reading
- Protein Structure and Function
- Båvik CO, Busch C, Eriksson U (Nov 1992). "Characterization of a plasma retinol-binding protein membrane receptor expressed in the retinal pigment epithelium". The Journal of Biological Chemistry. 267 (32): 23035–42. PMID 1331074.
- Hamel CP, Tsilou E, Harris E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM (Mar 1993). "A developmentally regulated microsomal protein specific for the pigment epithelium of the vertebrate retina". Journal of Neuroscience Research. 34 (4): 414–25. doi:10.1002/jnr.490340406. PMID 8474143.
- Tsilou E, Hamel CP, Yu S, Redmond TM (Oct 1997). "RPE65, the major retinal pigment epithelium microsomal membrane protein, associates with phospholipid liposomes" (PDF). Archives of Biochemistry and Biophysics. 346 (1): 21–7. doi:10.1006/abbi.1997.0276. PMID 9328280.
- Clinical and Genetic Studies
- Koenekoop RK, Lopez I, den Hollander AI, Allikmets R, Cremers FP (Jul 2007). "Genetic testing for retinal dystrophies and dysfunctions: benefits, dilemmas and solutions". Clinical & Experimental Ophthalmology. 35 (5): 473–85. doi:10.1111/j.1442-9071.2007.01534.x. PMID 17651254.
- Nicoletti A, Wong DJ, Kawase K, Gibson LH, Yang-Feng TL, Richards JE, Thompson DA (Apr 1995). "Molecular characterization of the human gene encoding an abundant 61 kDa protein specific to the retinal pigment epithelium". Human Molecular Genetics. 4 (4): 641–9. doi:10.1093/hmg/4.4.641. PMID 7633413.
- Hamel CP, Jenkins NA, Gilbert DJ, Copeland NG, Redmond TM (Apr 1994). "The gene for the retinal pigment epithelium-specific protein RPE65 is localized to human 1p31 and mouse 3". Genomics. 20 (3): 509–12. doi:10.1006/geno.1994.1212. PMID 8034329.
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP (Oct 1997). "Mutations in RPE65 cause Leber's congenital amaurosis". Nature Genetics. 17 (2): 139–41. doi:10.1038/ng1097-139. PMID 9326927.
- Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A (Oct 1997). "Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy". Nature Genetics. 17 (2): 194–7. doi:10.1038/ng1097-194. PMID 9326941.
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP (Mar 1998). "Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis". Proceedings of the National Academy of Sciences of the United States of America. 95 (6): 3088–93. doi:10.1073/pnas.95.6.3088. PMC 19699. PMID 9501220.
- Nicoletti A, Kawase K, Thompson DA (Mar 1998). "Promoter analysis of RPE65, the gene encoding a 61-kDa retinal pigment epithelium-specific protein". Investigative Ophthalmology & Visual Science. 39 (3): 637–44. PMID 9501877.
- Marlhens F, Griffoin JM, Bareil C, Arnaud B, Claustres M, Hamel CP (1999). "Autosomal recessive retinal dystrophy associated with two novel mutations in the RPE65 gene". European Journal of Human Genetics. 6 (5): 527–31. doi:10.1038/sj.ejhg.5200205. PMID 9801879.
- Ma JX, Zhang D, Laser M, Brownlee NA, Re GG, Hazen-Martin DJ, Redmond TM, Crouch RK (Jun 1999). "Identification of RPE65 in transformed kidney cells". FEBS Letters. 452 (3): 199–204. doi:10.1016/S0014-5793(99)00606-7. PMID 10386590.
- Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Héon E, Levin A, Jan J, Lam B, Carr RE, Franklin A, Radha S, Andorf JL, Sheffield VC, Stone EM (Apr 2000). "Mutation analysis of 3 genes in patients with Leber congenital amaurosis". Archives of Ophthalmology. 118 (4): 538–43. doi:10.1001/archopht.118.4.538. PMID 10766140.
- Simovich MJ, Miller B, Ezzeldin H, Kirkland BT, McLeod G, Fulmer C, Nathans J, Jacobson SG, Pittler SJ (Aug 2001). "Four novel mutations in the RPE65 gene in patients with Leber congenital amaurosis". Human Mutation. 18 (2): 164. doi:10.1002/humu.1168. PMID 11462243.
- Thompson DA, McHenry CL, Li Y, Richards JE, Othman MI, Schwinger E, Vollrath D, Jacobson SG, Gal A (Jan 2002). "Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively". American Journal of Human Genetics. 70 (1): 224–9. doi:10.1086/338455. PMC 384890. PMID 11727200.
- Felius J, Thompson DA, Khan NW, Bingham EL, Jamison JA, Kemp JA, Sieving PA (Jan 2002). "Clinical course and visual function in a family with mutations in the RPE65 gene". Archives of Ophthalmology. 120 (1): 55–61. doi:10.1001/archopht.120.1.55. PMID 11786058.
- Joseph B, Srinivasan A, Soumittra N, Vidhya A, Shetty NS, Uthra S, Kumaramanickavel G (Apr 2002). "RPE65 gene: multiplex PCR and mutation screening in patients from India with retinal degenerative diseases". Journal of Genetics. 81 (1): 19–23. doi:10.1007/BF02715866. PMID 12357075.
- Yzer S, van den Born LI, Schuil J, Kroes HY, van Genderen MM, Boonstra FN, van den Helm B, Brunner HG, Koenekoop RK, Cremers FP (Sep 2003). "A Tyr368His RPE65 founder mutation is associated with variable expression and progression of early onset retinal dystrophy in 10 families of a genetically isolated population". Journal of Medical Genetics. 40 (9): 709–13. doi:10.1136/jmg.40.9.709. PMC 1735582. PMID 12960219.