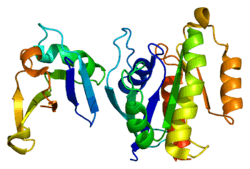
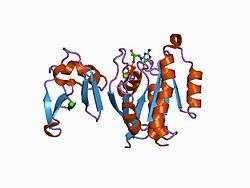
RAP1A
Ras-related protein Rap-1A is a protein that in humans is encoded by the RAP1A gene.[5]
Function
The product of this gene belongs to the family of Ras-related proteins. These proteins share approximately 50% amino acid identity with the classical RAS proteins and have numerous structural features in common. The most striking difference between RAP proteins and RAS proteins resides in their 61st amino acid: glutamine in RAS is replaced by threonine in RAP proteins. The product of this gene counteracts the mitogenic function of RAS because it can interact with RAS GAPs and RAF in a competitive manner. Two transcript variants encoding the same protein have been identified for this gene.[6]
Interactions
RAP1A has been shown to interact with:
gollark: Okay, so Kit was *not* bluffing?
gollark: ...
gollark: #6 is boring, I would never write that, I wrote #11.
gollark: Your code is broken, I didn't write #6.
gollark: Wow, kit was actually bluffing?
References
- GRCh38: Ensembl release 89: ENSG00000116473 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000068798 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Kawata M, Matsui Y, Kondo J, Hishida T, Teranishi Y, Takai Y (Dec 1988). "A novel small molecular weight GTP-binding protein with the same putative effector domain as the ras proteins in bovine brain membranes. Purification, determination of primary structure, and characterization". The Journal of Biological Chemistry. 263 (35): 18965–71. PMID 3143720.
- "Entrez Gene: RAP1A RAP1A, member of RAS oncogene family".
- Han L, Colicelli J (Mar 1995). "A human protein selected for interference with Ras function interacts directly with Ras and competes with Raf1". Molecular and Cellular Biology. 15 (3): 1318–23. doi:10.1128/mcb.15.3.1318. PMC 230355. PMID 7862125.
- Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A (Jun 1995). "The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue". Nature. 375 (6532): 554–60. doi:10.1038/375554a0. PMID 7791872.
- Hu CD, Kariya K, Okada T, Qi X, Song C, Kataoka T (Jan 1999). "Effect of phosphorylation on activities of Rap1A to interact with Raf-1 and to suppress Ras-dependent Raf-1 activation". The Journal of Biological Chemistry. 274 (1): 48–51. doi:10.1074/jbc.274.1.48. PMID 9867809.
- Okada T, Hu CD, Jin TG, Kariya K, Yamawaki-Kataoka Y, Kataoka T (Sep 1999). "The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases". Molecular and Cellular Biology. 19 (9): 6057–64. doi:10.1128/mcb.19.9.6057. PMC 84512. PMID 10454553.
- Boettner B, Govek EE, Cross J, Van Aelst L (Aug 2000). "The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin". Proceedings of the National Academy of Sciences of the United States of America. 97 (16): 9064–9. doi:10.1073/pnas.97.16.9064. PMC 16822. PMID 10922060.
- Nancy V, Callebaut I, El Marjou A, de Gunzburg J (Apr 2002). "The delta subunit of retinal rod cGMP phosphodiesterase regulates the membrane association of Ras and Rap GTPases". The Journal of Biological Chemistry. 277 (17): 15076–84. doi:10.1074/jbc.M109983200. PMID 11786539.
- Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC (May 2002). "The complex of Arl2-GTP and PDE delta: from structure to function". The EMBO Journal. 21 (9): 2095–106. doi:10.1093/emboj/21.9.2095. PMC 125981. PMID 11980706.
- Nancy V, Wolthuis RM, de Tand MF, Janoueix-Lerosey I, Bos JL, de Gunzburg J (Mar 1999). "Identification and characterization of potential effector molecules of the Ras-related GTPase Rap2". The Journal of Biological Chemistry. 274 (13): 8737–45. doi:10.1074/jbc.274.13.8737. PMID 10085114.
- Rebhun JF, Castro AF, Quilliam LA (Nov 2000). "Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction". The Journal of Biological Chemistry. 275 (45): 34901–8. doi:10.1074/jbc.M005327200. PMID 10934204.
- Castro AF, Rebhun JF, Clark GJ, Quilliam LA (Aug 2003). "Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner". The Journal of Biological Chemistry. 278 (35): 32493–6. doi:10.1074/jbc.C300226200. PMID 12842888.
- Yamamoto Y, Jones KA, Mak BC, Muehlenbachs A, Yeung RS (Aug 2002). "Multicompartmental distribution of the tuberous sclerosis gene products, hamartin and tuberin". Archives of Biochemistry and Biophysics. 404 (2): 210–7. doi:10.1016/s0003-9861(02)00300-4. PMID 12147258.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.