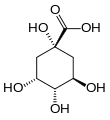
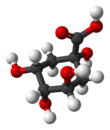
Quinic acid
Quinic acid is a cyclitol, a cyclic polyol, and a cyclohexanecarboxylic acid. It is a colorless solid that can be extracted from plant sources. Quinic acid is implicated in the perceived acidity of coffee.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexanecarboxylic acid | |||
| Preferred IUPAC name
(1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.976 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H12O6 | |||
| Molar mass | 192.17 g/mol | ||
| Density | 1.35 g/cm3 | ||
| Melting point | 168 °C (334 °F; 441 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Occurrence and preparation
The compound is obtained from cinchona bark, coffee beans, the bark of Eucalyptus globulus.[1] It is a constituent of the tara tannins.
Urtica dioica is another common source.
It is made synthetically by hydrolysis of chlorogenic acid. Quinic acid is also implicated in the perceived acidity of coffee.
History and biosynthesis
This substance was isolated for the first time in 1806 by French pharmacist Nicolas Vauquelin[2] and its transformation into hippuric acid by animal metabolism was studied by German chemist Eduard Lautemann in 1863.[3]
Its biosynthesis begins with the transformation of glucose into erythrose 4-phosphate. This four-carbon substrate is condensed with phosphoenol pyruvate to give the seven-carbon 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) by the action of a synthase. Two subsequent steps involving dehydroquinic acid synthase and a dehydrogenase afford the compound.[4]
Derived bicyclic lactones are called quinides. One example is 4-caffeoyl-1,5-quinide.
Dehydrogenation and oxidation of quinic acid affords gallic acid.[4]
Applications and medicinal activity
Quinic acid is used as an astringent.
This acid is a versatile chiral starting material for the synthesis of pharmaceuticals.[4] It is a building block in the preparation of the treatment of influenza A and B strains called Tamiflu.
Quinic acid is also thought to displace binding of the μ-opioid receptor antagonist naltrexone.
References
- Santos, Sónia A. O.; Freire, Carmen S. R.; Domingues, M. Rosário M.; Silvestre, Armando J. D.; Neto, Carlos Pascoal (2011). "Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry". Journal of Agricultural and Food Chemistry. 59 (17): 9386–93. doi:10.1021/jf201801q. PMID 21761864.
- L. N. Vauquelin (1806) "Expériences sur les diverses espèces de Quinquina" (Experiments on various species of Quinquina), Annales de Chimie, 59 : 113-169. Quinic acid is named on p. 167. From p. 167: "Concluons donc que cet acide est véritablement différent de tous ceux qui sont connus maintenant, et donnons-lui le nom d'acide kinique du mot quinquina, … " (Let us thus conclude that this acid is truly different from all those that are now known, and let us give it the name of quinic acid from the word quinquina, … )
- Lautemann, E. (1863) "Ueber die Reduction der Chinasäure zu Benzoësäure und die Verwandlung derselben in Hippursäure im thierischen Organismus" (On the reduction of quinic acid to benzoic acid and its transformation into hippuric acid in the animal organism), Annalen der Chemie, 125 : 9–13.
- Barco, Achille; Benetti, Simonetta; De Risi, Carmela; Marchetti, Paolo; Pollini, Gian P.; Zanirato, Vinicio (1997). "D(-)-Quinic Acid: a Chiron Store for Natural Product Synthesis". Tetrahedron: Asymmetry. 8: 3515–3545. doi:10.1016/S0957-4166(97)00471-0.CS1 maint: uses authors parameter (link)
Further reading
- "Quinic acid - chiral compounds from nature - Buchler quinine plant in Braunschweig,Germany". Quinic acid. Retrieved September 5, 2005.
- "Quinic acid". Fast Health. Retrieved September 5, 2005.
- "History of Xenobiotic Metabolism". History of Xenobiotic Metabolism. Archived from the original on April 12, 2005. Retrieved September 5, 2005.