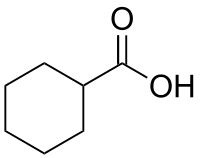
Cyclohexanecarboxylic acid
Cyclohexanecarboxylic acid is the organic compound with the formula C6H11CO2H. It is the carboxylic acid of cyclohexane. It is a colorless oil that crystallizes near room temperature.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Cyclohexanecarboxylic acid | |
| Other names
hexahydrobenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.465 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H12O2 | |
| Molar mass | 128.171 g·mol−1 |
| Appearance | white solid |
| Density | 1.0274 g/cm3 |
| Melting point | 30–31 °C (86–88 °F; 303–304 K) |
| Boiling point | 232–234 °C (450–453 °F; 505–507 K) |
| -83.24·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
It is prepared by hydrogenation of benzoic acid.
Cyclohexanecarboxylic acid is a precursor to the nylon-6 precursor caprolactam via its reaction with nitrosylsulfuric acid. It can also be oxidized to cyclohexene.[1]
Cyclohexanecarboxylic acid exhibits the reactions typical of carboxylic acids, including its conversion to the acid chloride cyclohexanecarbonyl chloride.[2][3]
Related compounds
Derivatives related to cyclohexanecarboxylic acid include:
- abscisic acid
- buciclic acid
- chlorogenic acid
- chorismic acid
- dicyclomine
- quinic acid
- shikimic acid
- tranexamic acid
gollark: I selected 8 stacks of white concrete, sent it 16 KST, and it did nothing.
gollark: <@173511059582222336> The concrete shop broke again.
gollark: I use osmarks.tk as *my* DNS.
gollark: I just use 8.8.4.4 as my backup, the other Google DNS.
gollark: OR IS IT?
External links
- Cyclohexanecarboxylic+Acids at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- Maki, Takao; Takeda, Kazuo. "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555..
- Turro, Nicholas J.; Leermakers, Peter A.; Vesley, George F. (1967). "Cyclohexylidenecyclohexane". Org. Synth. 47: 34. doi:10.15227/orgsyn.047.0034.
- Cope, Arthur C.; Ciganek, Engelbert (1959). "N,N-Dimethylcyclohexylmethylamine". Organic Syntheses. 39: 19. doi:10.15227/orgsyn.039.0019.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.