Quaternary compound
In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements.
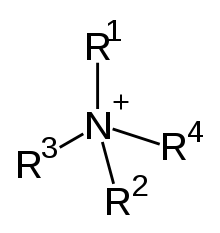
In another use of the term in organic chemistry, a quaternary compound is or has a cation consisting of a central positively charged atom with four substituents, especially organic (alkyl and aryl) groups, discounting hydrogen atoms.[1]
The best-known quaternary compounds are quaternary ammonium salts, having a nitrogen atom at the centre.[2] For example, in the following reaction, the nitrogen atom is said to be quaternized as it has gone from 3 to 4 substituents:
Other examples include substituted phosphonium salts (R4P+), substituted arsonium salts (R4As+) like arsenobetaine, as well as some arsenic-containing superconductors.[3] Substituted stibonium (R4Sb+)[4] and bismuthonium salts (R4Bi+) have also been described.[5]
See also
- Binary compound
- Ternary compound
- Onium ion
- Quaternary phase
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Onium compounds". doi:10.1351/goldbook.O04291
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Quaternary ammonium compounds". doi:10.1351/goldbook.Q05003
- Ren, Z. A.; Yang, J.; Lu, W.; Yi, W.; Shen, X. L.; Li, Z. C.; Che, G. C.; Dong, X. L.; Sun, L. L.; Zhou, F.; Zhao, Z. X. (2008). "Superconductivity in the iron-based F-doped layered quaternary compound Nd[O1 − x Fx]FeAs". EPL. 82 (5): 57002. arXiv:0803.4234. Bibcode:2008EL.....8257002R. doi:10.1209/0295-5075/82/57002.
- Widler, H. -J.; Schwarz, W.; Hausen, H. -D.; Weidlein, J. (1977). "Tetramethyl-Arsonium- und -Stibonium-Methylchlorometallate des Galliums und Indiums". Zeitschrift für anorganische und allgemeine Chemie. 435: 179. doi:10.1002/zaac.19774350124.
- Nicholas C. Norman (1997). Chemistry of arsenic, antimony, and bismuth. Springer Netherlands. p. 316. ISBN 0-7514-0389-X.