Pyridyne
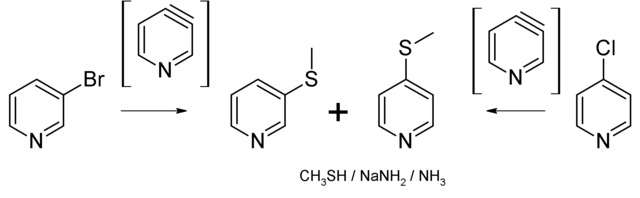
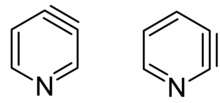
Pyridyne in chemistry is the pyridine analogue of benzyne.[1] This reactive intermediate is of some importance to scientific research. Pyridynes are the class of compounds sharing the pyridyne building motif. Two isomers exist, the 2,3-pyridine (2,3-didehydropyridine) and the 3,4-pyridyne (3,4-didehydropyridine). The reaction of 3-bromo-4-chloropyridine with furan and lithium amalgam gives 1,4-epoxy-dihydroquinoline through the 2,3-pyridyne intermediate. The reaction of 4-bromopyridine with sodium in liquid ammonia gives both 3-aminopyridine and 4-aminopyridine through the 3,4-pyridyne intermediate and an E1cB-elimination reaction.[2]
History
Pyridynes were first postulated by Levine and Leake in 1955.[3] In 1969 Zoltewicz and Nisi trapped 3,4-pyridyne in a reaction of 3-bromopyridine with methylmercaptan and sodium amide in ammonia. The methylthio and amino pyridines were found to be formed in the same ratio.[4]
In 1972 Kramer and Berry inferred the formation of 3,4-pyridyne in gas-phase photolysis of pyridine-3-diazonium-4-carboxylate via time-of-flight mass spectrometry. The dimer compound diazabiphenylene was detected.[5] In 1988 Nam and Leroy reported the matrix isolation (13K, Ar) of 3,4-pyridyne by photolysis of 3,4-pyridinedicarboxylic anhydride with the IR-spectrum revealing an acetylenic bond in the same way as ortho-benzyne.
Scope
Strategies involving pyridynes have been employed in the total syntheses of ellipticine [6][7] and (S)-Macrostomine [8]
References
- Handbook of Heterocyclic Chemistry, (2010) y, Alan R. Katritzky,Christopher A. Ramsden,J. Joule,Viktor V. Zhdankin
- Heterocyclic Chemistry, (2001) Malcolm Sainsbury
- Rearrangement in the Reaction of 3-Bromopyridine with Sodium Amide and Sodioacetophenone ROBERT LEVINE and WILLIAM W. LEAKE Science 27 May 1955: Vol. 121 no. 3152 p. 780 doi:10.1126/science.121.3152.780
- Trapping of 3,4-pyridyne by thiomethoxide ion in ammonia John A. Zoltewicz and Carlo Nisi The Journal of Organic Chemistry 1969 34 (3), 765-766doi:10.1021/jo01255a072
- Gaseous 3,4-pyridyne and the formation of diazabiphenylene Jerry Kramer and R. Stephen Berry Journal of the American Chemical Society 1972 94 (24), 8336-8347 doi:10.1021/ja00779a010
- Synthesis and Diels-Alder reactions of 1,3-dimethyl-4-(phenylsulfonyl)-4H-furo[3,4-b]indole. A new annulation strategy for the construction of ellipticine and isoellipticine Gordon W. Gribble, Mark G. Saulnier, Mukund P. Sibi, and Judy A. Obaza-Nutaitis The Journal of Organic Chemistry 1984 49 (23), 4518-4523 doi:10.1021/jo00197a039
- Total syntheses of ellipticine alkaloids and their amino analogues Original Research Article Tetrahedron, Volume 48, Issue 48, 27 November 1992, Pages 10645-10654 Chin-Kang Sha, Jeng-Fenn Yang doi:10.1016/S0040-4020(01)88360-5
- A Five-Step Synthesis of (S)-Macrostomine from (S)-Nicotine Monica F. Enamorado, Pauline W. Ondachi, and Daniel L. Comins Organic Letters 2010 12 (20), 4513-4515 doi:10.1021/ol101887b