Pyrazine
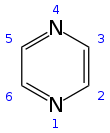
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pyrazine | |||
| Other names
1,4-Diazabenzene, p-Diazine, 1,4-Diazine, Paradiazine, Piazine, UN 1325 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.480 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H4N2 | |||
| Molar mass | 80.09 g/mol | ||
| Appearance | White crystals | ||
| Density | 1.031 g/cm3 | ||
| Melting point | 52 °C (126 °F; 325 K) | ||
| Boiling point | 115 °C (239 °F; 388 K) | ||
| Soluble | |||
| Acidity (pKa) | 0.37[1] (protonated pyrazine) | ||
| -37.6·10−6 cm3/mol | |||
| Hazards | |||
| R-phrases (outdated) | R11, R36/37/38 | ||
| S-phrases (outdated) | S16, S26, S36 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) c.c. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Pyrazine and a variety of alkylpyrazines are flavor and aroma compounds found in baked and roasted goods. Tetramethylpyrazine (also known as ligustrazine) is reported to scavenge superoxide anion and decrease nitric oxide production in human polymorphonuclear leukocytes.[2]
Synthesis
Many methods exist for the organic synthesis of pyrazine and its derivatives. Some of these are among the oldest synthesis reactions still in use.
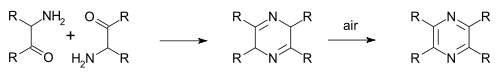
In the Staedel–Rugheimer pyrazine synthesis (1876) 2-chloroacetophenone is reacted with ammonia to the amino ketone, then condensed and then oxidized to a pyrazine.[3] A variation is the Gutknecht pyrazine synthesis (1879) also based on this selfcondensation, but differing in the way the alpha-ketoamine is synthesised.[4][5]
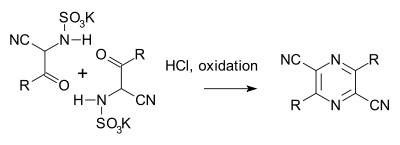
The Gastaldi synthesis (1921) is another variation:[6][7]
See also
- Alkylpyrazines
- Benzene, an analog without the nitrogen atoms
- Methoxypyrazines
- Pyridazine, an analog with the second nitrogen atom in position 2
- Pyridine, an analog with only one nitrogen atom
- Pyrimidine, an analog with the second nitrogen atom in position 3
- Simple aromatic rings
References
- Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- Zhang, Zhaohui (2003). "Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes". Life Sciences. 72 (22): 2465–2472. doi:10.1016/S0024-3205(03)00139-5.
- Ueber die Einwirkung von Ammoniak auf Chloracetylbenzol (pp. 563–564) W. Staedel, L. Rügheimer doi:10.1002/cber.187600901174 Berichte der deutschen chemischen Gesellschaft Volume 9, Issue 1, pp. 563–564, 1876
- Mittheilungen Ueber Nitrosoäthylmethylketon H. Gutknecht Berichte der deutschen chemischen Gesellschaft Volume 12, Issue 2 , pp. 2290–2292, 1879 doi:10.1002/cber.187901202284
- Heterocyclic chemistry T.L. Gilchrist ISBN 0-582-01421-2
- G. Gastaldi, Gazz. Chim. Ital. 51, (1921) 233
- Amines: Synthesis, Properties and Applications Stephen A. Lawrence 2004 Cambridge University Press ISBN 0-521-78284-8