Alkylpyrazine
Alkylpyrazines are chemical compounds based on pyrazine with different substitution patterns. Some alkylpyrazines are naturally occurring highly aromatic substances which often have a very low odor threshold[1] and contribute to the taste and aroma of various foods including cocoa, baked goods, coffee and wines. [2][3] Alkylpyrazines are also formed during the cooking of some foods via Maillard reactions.[1]
Examples
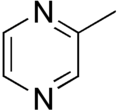 2-Methylpyrazine (found in roasted sesame)
2-Methylpyrazine (found in roasted sesame)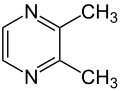 2,3-Dimethylpyrazine (found in roasted sesame)
2,3-Dimethylpyrazine (found in roasted sesame)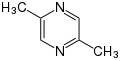 2,5-Dimethylpyrazine (asparagus, tea, crispbread, malt, shrimp, soy, squid, Swiss chease, bread, roasted sesame)
2,5-Dimethylpyrazine (asparagus, tea, crispbread, malt, shrimp, soy, squid, Swiss chease, bread, roasted sesame)- 2,3,5,6-Tetramethylpyrazine (nattō, fermented cocoa beans)
2-Methylpyrazine
2-Methylpyrazine was the volatile odorant found at the highest concentration in roasted sesame seed oil.[4]
2,3-Dimethylpyrazine
2,3-Dimethylpyrazine is a component of the aroma of roasted sesame seeds.[4]
2,5-Dimethylpyrazine
2,5-Dimethylpyrazine is used as flavor additive and odorant in foods such as cereals[5] and products such as cigarettes. It occurs naturally in asparagus, black or green tea, crispbread, malt, raw shrimp, soya, squid, Swiss cheeses, and wheat bread.[5] It's also found in roasted sesame.[4] The chemical formula is C6H8N2.[5]
2,6-Dimethylpyrazine
2,6-Dimethylpyrazine is also used as flavor additive and odorant in foods such as cereals[6] and products such as cigarettes. It occurs naturally in baked potato, black or green tea, crispbread, French fries, malt, peated malt, raw asparagus, roasted barley, roasted filberts or pecans, squid, wheat bread, wild rice (Zizania aquatica), and wort.[6] It's also found in roasted sesame.[4] The chemical formula is C6H8N2.[6]
2,3,5,6-Tetramethylpyrazine
Tetramethylpyrazine (ligustrazine) is found in nattō and in fermented cocoa beans.[7]
See also
References
- Susan M. Fors; Bertil K. Olofsson (1985). "Alkylpyrazines, volatiles formed in the Maillard reaction. I. Determination of odour detection thresholds and odour intensity functions by dynamic olfactometry". Chemical Senses. 10 (3): 287–296. doi:10.1093/chemse/10.3.287.
- Zhao, Chao; Cao, Hui; Xiao, Jianbo (2020). "Pyrazines in Food". Handbook of Dietary Phytochemicals. Springer: 1–25. doi:10.1007/978-981-13-1745-3_44-1. Retrieved 5 May 2020.
- Mihara, Satoru; Masuda, Hideki (1988). "Structure-odor relationships for disubstituted pyrazines". Journal of Agricultural and Food Chemistry. 36 (6): 1242–7. doi:10.1021/jf00084a029.
- Shimoda, M., Shiratsuchi, H., Nakada, Y., Wu, Y., & Osajima, Y. (1996). "Identification and Sensory Characterization of Volatile Flavor Compounds in Sesame Seed Oil". Journal of Agricultural and Food Chemistry. 44 (12): 3909–3912. doi:10.1021/jf960115f.CS1 maint: uses authors parameter (link)
- "2,5-DIMETHYLPYRAZINE". PubChem Compound Database; CID=31252. National Center for Biotechnology Information. Retrieved 2017-01-21.
- "2,6-DIMETHYLPYRAZINE". PubChem Compound Database; CID=7938. National Center for Biotechnology Information. Retrieved 2017-01-21.
- Effect of mass and turning time on free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. P Hashim, J Selamat, S Muhammad, S Kharidah and A Ali, Journal of the Science of Food and Agriculture, 1998, Volume 78, pages 543–550. doi:10.1002/(SICI)1097-0010(199812)78:4<543::AID-JSFA152>3.0.CO;2-2