Pterocarpan
Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes (i.e. 6H-[1]benzofuro[3,2-c]chromene skeleton) which can be formed by coupling of the B ring to the 4-one position.[1]
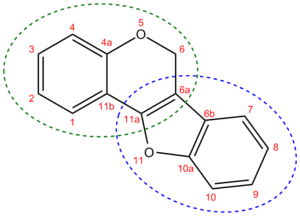
Pterocarpan chemical structure. It consists of a 1-benzofuran moiety (dotted blue circle) fused to a 2H-chromene moiety (dotted green circle). The systematic name for it is 6H-[1]benzofuro[3,2-c]chromene. The new numbering of the resulting moiety is shown with red numbers.
2'-hydroxyisoflavone reductase is the enzyme responsible for the conversion in Cicer arietinum[2] and glyceollin synthase for the production of glyceollins, phytoalexins in soybean.[3]
Known compounds
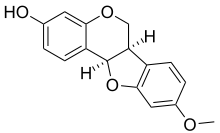
Medicarpin chemical structure
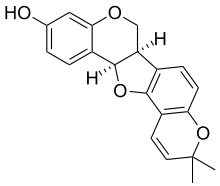
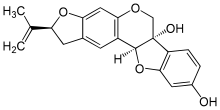
- Bitucarpin A and B, isolated from the aerial parts of Mediterranean plants Bituminaria morisiana and Bituminaria bituminosa[4]
- Erybraedin A and B, isolated from the stems of Erythrina subumbrans[5] and C, isolated from the leaves of Bituminaria morisiana[6]
- Erythrabyssin II, erystagallin A, erythrabissin-1, and erycristagallin isolated from the stems of Erythrina subumbrans[5]
- Glycinol, glyceollidin I and II, glyceollins (glyceollin I, II, III and IV), found in the soybean (Glycine max)[7]
- Glycyrrhizol A, isolated from the root of the Chinese licorice plant (Glycyrrhiza uralensis)
- Maackiain, isolated from the roots of Maackia amurensis subsp. Buergeri[8]
- Medicarpin, found in Medicago truncatula
- Morisianine, isolated from the seeds of Bituminaria morisiana[9]
- Orientanol A, isolated from the wood of Erythrina orientalis[10]
- Phaseolin, found in French bean seeds[11]
- Pisatin, found in Pisum sativum[12]
- Striatine, isolated from aerial parts of Mundulea striata[13]
- Trifolirhizin, found in Sophora flavescens
gollark: If you want "simple", how about, I don't know, lisp?
gollark: "Costless" how?
gollark: I'd partly agree, but that doesn't mean ALL ABSTRACTION is hard to use.
gollark: If we accept your ridiculous "simple to implement means easy" thing, then machine code is easier than assembly, and... CPU microcode? is easier than machine code.
gollark: Assembly is an abstraction over machine code.
References
- Pterocarpans on the National Library of Medicine - Medical Subject Headings
- Isolation of NADPH:isoflavone oxidoreductase, a new enzyme of Pterocarpan phytoalexin biosynthesis in cell suspension cultures of Cicer arietinum. Karin Tiemann, Walter Hinderer and Wolfgang Barz, FEBS Letters, Volume 213, Issue 2, 23 March 1987, Pages 324-328, doi:10.1016/0014-5793(87)81515-6
- Welle R, Grisebach H (1988). "Induction of phytoalexin synthesis in soybean: enzymatic cyclization of prenylated pterocarpans to glyceollin isomers". Arch. Biochem. Biophys. 263 (1): 191–8. doi:10.1016/0003-9861(88)90627-3. PMID 3369863.
- Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Dedicated to the memory of Professor Jeffrey B. Harborne. Luisa Pistelli, Cecilia Noccioli, Giovanni Appendino, Federica Bianchi, Olov Sterner and Mauro Ballero, Phytochemistry, Volume 64, Issue 2, September 2003, Pages 595-598, doi:10.1016/S0031-9422(03)00190-0
- Antibacterial Pterocarpans from Erythrina subumbrans. Thitima Rukachaisirikul, Phongsak Innok, Nuntana Aroonrerk, Woraluk Boonamnuaylap, Saranya Limrangsun, Chanakan Boonyon, Umpawan Woonjina and Apichart Suksamrarn, Journal of Ethnopharmacology, Volume 110, Issue 1, 1 March 2007, Pages 171-175, doi:10.1016/j.jep.2006.09.022
- New cytotoxic prenylated isoflavonoids from Bituminaria morisiana. Cottiglia Filippo, Casu Laura, Bonsignore Leonardo, Casu Mariano, Floris Costantino, Leonti Marco, Gertsch Juerg and Heilmann Jörg, Planta medica 71 (3) (2005), pp. 254-260
- Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy. M. Carla Zimmermann, Syreeta L. Tilghman, Stephen M. Boué, Virgilio A. Salvo, Steven Elliott, K. Y. Williams, Elena V. Skripnikova, Hasina Ashe, Florastina Payton-Stewart, Lyndsay Vanhoy-Rhodes, Juan Pablo Fonseca, Cynthia Corbitt, Bridgette M. Collins-Burow, Melanie H. Howell, Michelle Lacey, Betty Y. Shih, Carol Carter-Wientjes, Thomas E. Cleveland, John A. McLachlan, Thomas E. Wiese, Barbara S. Beckman and Matthew E. Burow, JPET January 2010 vol. 332 no. 1, doi:10.1124/jpet.109.160382
- A prenylated flavanone from roots of Maackia amurensis subsp. Buergeri. Nobuyasu Matsuura, Rie Nakai, Munekazu Iinuma, Toshiyuki Tanaka and Kenichro Inoue, Phytochemistry, Volume 36, Issue 1, May 1994, Pages 255-256, doi:10.1016/S0031-9422(00)97051-1
- A pterocarpan from the seeds of Bituminaria morisiana. Marco Leonti, Laura Casu, Jurg Gertsch, Leonardo Bonsignore, Costantino Floris, Mariano Casu and Filippo Cottiglia, J Nat Med (2010) 64:354–357, doi:10.1007/s11418-010-0408-7
- A pterocarpan from Erythrina orientalis. Hitoshi Tanaka, Toshihiro Tanaka and Hideo Etoh, Phytochemistry, Volume 45, Issue 1, May 1997, Pages 205-207, doi:10.1016/S0031-9422(96)00841-2
- Physicochemical and structural studies of phaseolin from French bean seed. R. J. Blagrove, P. M. Colman, G. G. Lilley, A. Van Donkelaar and E. Suzuki, Plant Foods For Human Nutrition (formerly Qualitas Plantarum), Volume 33, Numbers 2-3, 227-229, doi:10.1007/BF01091313
- Pisatin: an Antifungal Substance from Pisum sativum L. Dawn R. Perrin and W. Bottomley, Nature 191, 76 - 77 (1 July 1961), doi:10.1038/191076a0
- A prenylated pterocarpan from Mundulea striata. Frédéric Manjary, Alain Petitjean, Jean-Yves Conan, Marie Thérèse Martin, François Frappier, Philippe Rasoanaivo and Suzanne Ratsimamanga-Urverg, Phytochemistry, Volume 33, Issue 2, 13 May 1993, Pages 515-517, doi:10.1016/0031-9422(93)85554-5
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.