Pterin
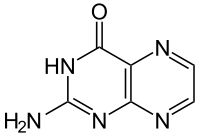
Pterin is a heterocyclic compound composed of a pteridine ring system, with a "keto group" (a lactam) and an amino group on positions 4 and 2 respectively. It is structurally related to the parent bicyclic heterocycle called pteridine. Pterins, as a group, are compounds related to pterin with additional substituents. Pterin itself is of no biological significance.
 | |
| Names | |
|---|---|
| IUPAC names
2-aminopteridin-4(3H)-one (one of five tautomers) | |
| Other names
Pteridoxamine Pterine 4-Oxopterin 2-Amino-4-pteridone 2-Amino-4-hydroxypteridine 2-Amino-4-oxopteridine 2-aminopteridin-4-ol 2-Amino-4-pteridinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.091 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5N5O | |
| Molar mass | 163.137 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pterins were first discovered in the pigments of butterfly wings[1] (hence the origin of their name, from the Greek pteron (πτερόν),[2] wing) and perform many roles in coloration in the biological world.
Pterin cofactors
One important family of pterin derivatives are Folates. Folates are pterins that contain p-aminobenzoic acid connected to the methyl group at position 6 of the pteridine ring system (known as pteroic acid) conjugated with one or more L-glutamates. They participate in numerous biological group transfer reactions. Folate-dependent biosynthetic reactions include the transfer of methyl groups from 5-methyltetrahydrofolate to homocysteine to form L-methionine, and the transfer of formyl groups from 10-formyltetrahydrofolate to L-methionine to form N-formylmethionine in initiator tRNAs. Folates are also essential for the biosynthesis of purines and one pyrimidine.
Biosynthetic precursor
Substituted pteridines are intermediates in the biosynthesis of dihydrofolic acid in many microorganisms.[3] The enzyme dihydropteroate synthetase converts pteridine and 4-aminobenzoic acid to dihydrofolic acid in the presence of glutamate. The enzyme dihydropteroate synthetase is inhibited by sulfonamide antibiotics.
Molybdopterin is a cofactor found in virtually all molybdenum and tungsten-containing proteins.[4]
Tetrahydrobiopterin
Tetrahydrobiopterin, the major unconjugated pterin in vertebrates, is involved in three families of enzymes that effect hydroxylation. The aromatic amino acid hydroxylases include phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylases. They are involved in the synthesis of neurotransmitters catecholamine and serotonin. Tetrahydrobiopterin is also required for the functioning of alkylglycerol monooxygenase, whereby monoalkylglycerols are broken down to glycerol and an aldehyde. In the synthesis of nitric oxide the pterin-dependent nitric oxide synthase converts arginine to N-hydroxy derivative, which in turn releases NO.[5]
Biosynthesis
The biosynthesis of pterins begins with guanosine triphosphate (GTP), which is the substrate for GTP cyclohydrolase I.[6] The enzyme is found in both prokaryotes and eukaryotes.
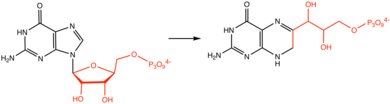
Molybdopterin biosynthesis occurs in four steps: (i) the radical-mediated cyclization of nucleotide, guanosine 5'-triphosphate (GTP), to (8S)‑3 ́,8‐cyclo‑7,8‑dihydroguanosine 5 ́‑triphosphate (3 ́,8‑cH2GTP), (ii) the formation of cyclic pyranopterin monophosphate (cPMP) from the 3 ́,8‑cH2GTP, (iii) the conversion of cPMP into molybdopterin (MPT), (iv) the insertion of molybdate into MPT to form Moco. The human enzymes are indicated in parenthesis.[7]

Other pterins
Pterin can exist in many different forms in nature depending on its function. Tetrahydrobiopterin (BH4), the major unconjugated pteridine in vertebrates, is a co-factor in the hydroxylation of aromatic compounds and synthesis of nitric oxide. Molybdopterin is a substituted pteridine that binds molybdenum to yield redox cofactors involved in biological hydroxylations, reduction of nitrate, and respiratory oxidation. Tetrahydromethanopterin is used in methanogenic organisms.[8] Cyanopterin is a glycosylated version of pteridine of unknown function in cyanobacteria.
_male.jpg)
Tautomers
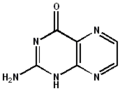 2-aminopteridin-4(1H)-one
2-aminopteridin-4(1H)-one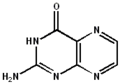 2-aminopteridin-4(3H)-one
2-aminopteridin-4(3H)-one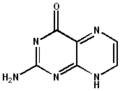 2-aminopteridin-4(8H)-one
2-aminopteridin-4(8H)-one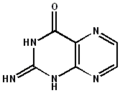 2-imino-2,3-dihydropteridin-4(1H)-one
2-imino-2,3-dihydropteridin-4(1H)-one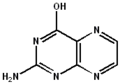 2-aminopteridin-4-ol
2-aminopteridin-4-ol
See also
References
- Wijnen, B.; Leertouwer, H. L.; Stavenga, D. G. (2007). "Colors and pterin pigmentation of pierid butterfly wings" (PDF). Journal of Insect Physiology. 53 (12): 1206–17. doi:10.1016/j.jinsphys.2007.06.016. PMID 17669418.
- πτερόν. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- Voet, D.; Voet, J.G. (2004). Biochemistry (3rd ed.). John Wiley & Sons. ISBN 0-471-39223-5
- Feirer, Nathan; Fuqua, Clay (2017-01-01). "Pterin function in bacteria". Pteridines. 28 (1). doi:10.1515/pterid-2016-0012. ISSN 2195-4720.
- Werner, Ernst R. (2013-01-01). "Three classes of tetrahydrobiopterin-dependent enzymes". Pteridines. 24 (1). doi:10.1515/pterid-2013-0003. ISSN 2195-4720.
- J. Rebelo, G. Auerbach, G Bader, A. Bracher, H. Nar, C. Hösl, N. Schramek, J. Kaiser, A. Bacher, R. Huber, M. Fischer (2003). "Biosynthesis of Pteridines. Reaction Mechanism of GTP Cyclohydrolase I". Journal of Molecular Biology. 326 (2): 503–516. doi:10.1016/S0022-2836(02)01303-7. PMID 12559918.CS1 maint: uses authors parameter (link)
- Schwarz, G. & Mendel, R. R. (2006). "Molybdenum cofactor biosynthesis and molybdenum enzymes". Annual Review of Plant Biology. 57: 623–647. doi:10.1146/annurev.arplant.57.032905.105437. PMID 16669776.
- Schwarz, Guenter; Mendel, Ralf R.; Ribbe, Markus W. (2009). "Molybdenum cofactors, enzymes and pathways". Nature. 460 (7257): 839–847. Bibcode:2009Natur.460..839S. doi:10.1038/nature08302. PMID 19675644.CS1 maint: uses authors parameter (link)
- B. Wijnen, H. L. Leertouwer, D. G.Stavenga (2007). "Colors and pterin pigmentation of pierid butterfly wings" (PDF). Journal of Insect Physiology. 53 (12): 1206–1217. doi:10.1016/j.jinsphys.2007.06.016. PMID 17669418.CS1 maint: uses authors parameter (link)