Pseudobulbar affect
Pseudobulbar affect (PBA), or emotional incontinence, is a type of emotional disturbance characterized by uncontrollable episodes of crying and/or laughing, or other emotional displays. PBA occurs secondary to a neurologic disorder or brain injury. Patients may find themselves crying uncontrollably at something that is only moderately sad, being unable to stop themselves for several minutes. Episodes may also be mood-incongruent: a patient may laugh uncontrollably when angry or frustrated, for example. Sometimes, the episodes may switch between emotional states, resulting in the patient crying uncontrollably before dissolving into fits of laughter.
| Pseudobulbar affect | |
|---|---|
| Other names | Emotional incontinence |
| Specialty | Psychiatry, neurology |
| Causes | Brain trauma, ALS |
The pseudobulbar affect, also referred to as emotional lability, should not be confused with labile mood or labile emotions that stem from emotional instability – affective dysregulation – commonly seen in personality disorders, such as borderline personality disorder.[1]
Signs and symptoms
The cardinal feature of the disorder is a pathologically lowered threshold for exhibiting the behavioral response of laughter, crying, or both. An affected individual exhibits episodes of laughter and/or crying without an apparent motivating stimulus or in response to stimuli that would not have elicited such an emotional response before the onset of their underlying neurologic disorder. In some patients, the emotional response is exaggerated in intensity but is provoked by a stimulus with an emotional valence congruent with the character of the emotional display. For example, a sad stimulus provokes a pathologically exaggerated weeping response instead of a sigh, which the patient normally would have exhibited in that particular instance.
However, in some other patients, the character of the emotional display can be incongruent with, and even contradictory to, the emotional valence of the provoking stimulus or may be incited by a stimulus with no clear valence. For example, a patient may laugh in response to sad news or cry in response to stimuli with no emotional undertone, or, once provoked, the episodes may switch from laughing to crying or vice versa.[2]
The symptoms of PBA can be severe, with persistent and unremitting episodes.[3] Characteristics include:
- The onset can be sudden and unpredictable, and has been described by some patients as coming on like a seizure;
- The outbursts have a typical duration of a few seconds to several minutes; and,
- The outbursts may happen several times a day.
Many people with neurologic disorders exhibit uncontrollable episodes of laughing, crying, or both that are either exaggerated or contradictory to the context in which they occur. Where patients have significant cognitive deficits (e.g., Alzheimer's) it can be unclear whether it is true PBA as opposed to a grosser form of emotional dysregulation, but patients with intact cognition often report the symptom as disturbing. Patients report that their episodes are at best only partially amenable to voluntary control, and unless they experience a severe change of mental status, they often have insight into their problem and judge their emotional display as inappropriate and out of character. The clinical effect of PBA can be severe, with unremitting and persistent symptoms that can be disabling to patients, and may significantly affect quality of life for caregivers.
Social impact
While not as profoundly disabling as the physical symptoms of these diseases, PBA may significantly influence individuals' social functioning and their relationships with others. Such sudden, frequent, extreme, uncontrollable emotional outbursts may lead to social withdrawal and interfere with activities of daily living, social and professional pursuits, and reduce overall healthcare. For example, patients with ALS and MS are often cognitively normal. However, the appearance of uncontrollable emotions is commonly associated with many additional neurological disorders such as attention deficit/hyperactivity disorder,[4] Parkinson's disease,[5] cerebral palsy,[6] autism,[7] epilepsy,[8] and migraines.[9] This may lead to avoidance of social interactions for the patient, which in turn impairs their coping mechanisms and their careers.[3][10][11][12][13]
Depression
PBA may often be misdiagnosed as clinical depression; however, many clear distinctions exist.
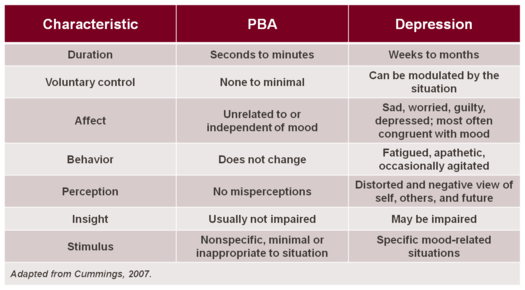
In depression and grief syndromes, crying is typically a sign of sadness, whereas the pathological displays of crying which occur in PBA are often in contrast to the underlying mood, or greatly in excess of the mood or eliciting stimulus. In addition, a key to differentiating depression from PBA is duration: PBA episodes are sudden, occurring in a brief episodic manner, while crying in depression is a more sustained presentation and closely relates to the underlying mood state. The level of control that one has over the crying episodes in PBA is minimal or nonexistent, whereas for those suffering from depression, the emotional expression (typically crying) can be modulated by the situation. Similarly, the trigger for episodes of crying in patients with PBA may be nonspecific, minimal or inappropriate to the situation, but in depression the stimulus is specific to the mood-related condition. These differences are outlined in the adjacent Table.
In some cases, depressed mood and PBA may co-exist. Since depression is one of the most common emotional changes in patients with neurodegenerative disease or post-stroke sequelae, it is often comorbid with PBA. Comorbidity implies that depression is distinct from PBA and is not necessary for, nor does it exclude, a diagnosis of PBA.[14]
Causes
The specific pathophysiology involved in this frequently debilitating condition is still under investigation; the primary pathogenic mechanisms of PBA remain controversial.[15] One hypothesis, established by early researchers such as Wilson and Oppenheim, placed emphasis on the role of the corticobulbar pathways in modulating emotional expression in a top-down model, and theorized that PBA occurs when bilateral lesions in the descending corticobulbar tract cause failure of voluntary control of emotion, which leads to the disinhibition, or release, of laughing/crying centers in the brainstem.[16] Other theories implicate the prefrontal cortex.[17]
Secondary condition
PBA is a condition that occurs secondary to neurological disease or brain injury, and is thought to result from disruptions of neural networks that control the generation and regulation of motor output of emotions. PBA is most commonly observed in people with neurologic injuries such as traumatic brain injury (TBI) and stroke,[16][18] and neurologic diseases such as dementias including Alzheimer's disease, attention deficit/hyperactivity disorder (ADHD),[4][19] multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), PANDAS in children and adults, and Parkinson's disease (PD). It has been reported as a symptom of hyperthyroidism, Graves' Disease, or hypothyroidism in combination with depression.[20]
PBA has also been observed in association with a variety of other brain disorders, including brain tumors, Wilson's disease, syphilitic pseudobulbar palsy, and various encephalitides. Rarer conditions associated with PBA include gelastic epilepsy, dacrystic epilepsy, central pontine myelinolysis, olivopontinocerebellar atrophy, lipid storage diseases, chemical exposure (e.g., nitrous oxide and insecticides), fou rire prodromique, and Angelman syndrome.
It is hypothesized that these primary neurologic injuries and diseases affect chemical signaling in the brain, which in turn disrupts the neurologic pathways that control emotional expression.[21][22][23]
Stroke
PBA is one of the most frequently reported post-stroke behavioral syndromes, with a range of reported prevalence rates from 28% to 52%.[24][25][26] The higher prevalence rates tend to be reported in stroke patients who are older and/or who have a history of prior stroke.[27][28] The relationship between post-stroke depression and PBA is complicated, because the depressive syndrome also occurs with high frequency in stroke survivors. Post-stroke patients with PBA are more depressed than post-stroke patients without PBA, and the presence of a depressive syndrome may exacerbate the weeping side of PBA symptoms.[24][29]
Multiple sclerosis
Recent studies suggest that approximately 10% of patients with multiple sclerosis (MS) will experience at least one episode of emotional lability.[30][31] PBA is generally associated with later stages of the disease (chronic progressive phase).[26] PBA in MS patients is associated with more severe intellectual deterioration, physical disability, and neurological disability.[32]
Amyotrophic lateral sclerosis
A study designed specifically to survey for prevalence found that 49% of patients with amyotrophic lateral sclerosis (ALS) also had PBA.[10] PBA does not appear to be associated with duration of ALS.[33][34] It is a symptom of ALS that many patients are unaware of and do not receive information about from their physician.[35]
Traumatic brain injury
One study of 301 consecutive cases in a clinic setting reported a 5% prevalence. PBA occurred in patients with more severe head injury, and coincided with other neurological features suggestive of pseudobulbar palsy.[36]
The Brain Injury Association of America (BIAA) indicates that approximately 80% of survey respondents experience symptoms of PBA.[37] Results from a recent investigation estimate the prevalence of PBA associated with traumatic brain injury to exceed more than 55% of survivors.[38]
Treatment
Education of patients, families, and caregivers is an important component of the appropriate treatment of PBA. Crying associated with PBA may be incorrectly interpreted as depression; laughter may be embarrassing. It is therefore critical for families and caregivers to recognize the pathological nature of PBA and the reassurance that this is an involuntary syndrome that is manageable. Traditionally, antidepressants such as sertraline,[39] fluoxetine,[40] citalopram,[41] nortriptyline[42] and amitriptyline[43] have been prescribed with some efficacy.
Medication
Dextromethorphan hydrobromide affects the signals in the brain that trigger the cough reflex. It is used as a cough suppressant, although it can sometimes be used, medicinally, as a pain reliever, and is also used as a recreational drug.[44]
Quinidine sulfate affects the way the heart beats, and is generally used in people with certain heart rhythm disorders. It is also used to treat malaria.[45] Quinidine sulfate, as a metabolic inhibitor, "increases plasma levels of dextromethorphan by competitively inhibiting cytochrome P450 2D6, which catalyzes a major biotransformation pathway for dextromethorphan," enabling therapeutic dextromethorphan concentrations.[46]
Dextromethorphan/quinidine is a combination of these two generic drugs, and is the first FDA-approved drug for the treatment of PBA, approved on October 29, 2010.[47]
For this multicenter study, the "Objectives...[were] to evaluate the safety, tolerability, and efficacy of two different doses of AVP-923 [Dextromethorphan/quinidine combination]...when compared to placebo."[48] The conditions and results of that study are as follows:
At one study site, a total of 326 participants received one of three dose options. "METHODS: In a 12-week randomized, double-blind trial, ALS and MS patients with clinically significant PBA" were given a twice-daily dose of one of the following:
- placebo (N=109)
- dextromethorphan hydrobromide 30 mg/quinidine sulfate 10 mg (N=110)
- Nuedexta – dextromethorphan hydrobromide 20 mg/quinidine sulfate 10 mg (N=107)
283 patients (86.8%) completed the study. The number of PBA episodes (laughing and crying) were 47% and 49% lower (based on the trial's outcome measures), respectively, for the drug-combination options than for the placebo. The "mean CNS-LS scores" decreased by 8.2 points for both drug-combination options, vs a decrease of 5.7 points for the placebo.
Overall, the trial showed a statistically significant benefit from taking a combination of dextromethorphan and quinidine, with both dosages being safe and well tolerated. For a secondary objective measuring a participant's "perceived health status...measuring eight health concepts: vitality, physical functioning, bodily pain, general health perceptions, physical role-, emotional role-, social role functioning, and mental health," the higher dosage showed improvement, especially on measures of social functioning and mental health.[48][49]
Epidemiology
Prevalence estimates place the number of people with PBA between 1.5 and 2 million in the United States alone, which would be less than 1% of the U.S. population even at the high end of the estimate. Some argue that the number is probably higher and that clinicians underdiagnose PBA.[50] However, the prevalence estimate of 2 million is based on an online survey. Self-selected computer-savvy patients in at-risk groups evaluated their own symptoms and submitted their self-diagnoses. No doctor or clinic confirmed the data. Motivation to participate could have been influenced by the presence of symptoms, which would have skewed the results. The actual prevalence could very well be quite a bit lower than estimated.[51]
History
The Expression of the Emotions in Man and Animals by Charles Darwin was published in 1872.[52] In Chapter VI, "Special Expressions of Man: Suffering and Weeping", Darwin discusses cultural variations in the acceptability of weeping and the wide differences in individual responses to suffering. The chapter contains the following sentence:
We must not, however, lay too much stress on the copious shedding of tears by the insane, as being due to the lack of all restraint; for certain brain-diseases, as hemiplegia, brain-wasting, and senile decay, have a special tendency to induce weeping.[53]
Terminology
Historically, there have been a variety of terms used for the disorder, including pseudobulbar affect, pathological laughter and crying, emotional lability, emotionalism, emotional dysregulation, or more recently, involuntary emotional expression disorder.[14] The term pseudobulbar (pseudo- + bulbar) came from the idea that the symptoms seemed similar to those caused by a bulbar lesion (that is, a lesion in the medulla oblongata).
Terms such as forced crying, involuntary crying, pathological emotionality, and emotional incontinence have also been used, although less frequently.[3]
In popular culture
Signs of pseudobulbar affect were featured as affecting the title character of the 2019 film Joker,[54] and are said to be what Joaquin Phoenix used as inspiration for his character's titular laugh.
In the 2019 movie Parasite, the character named Ki-woo suffers a head trauma, and although it is not clearly mentioned that he's affected by pseudobulbar affect, he mentions not being able to stop laughing when thinking about all the events that occur in the movie.
In the medical television show House, season 7, episode 8 (Small Sacrifices), the character Ramon Silva, played by Kuno Becker displays pseudobulbar affect, with uncontrollable incongruent laughter, while suffering from Marburg variety of Multiple sclerosis.
See also
References
- mentalhealth.com/home/dx/borderlinepersonality.html
- Parvizi J, Archiniegas DB, Bernardini GL, Hoffman MW, et al. (2006). "Diagnosis and management of pathological laughter and crying". Mayo Clinic Proceedings. 81 (11): 1482–1486. doi:10.4065/81.11.1482. PMID 17120404.
- Dark FL, McGrath JJ, Ron MA; McGrath; Ron (1996). "Pathological laughing and crying". Australian and New Zealand Journal of Psychiatry. 30 (4): 472–479. doi:10.3109/00048679609065020. PMID 8887697.CS1 maint: multiple names: authors list (link)
- Skirrow, Caroline; Asherson, Philip (2013). "Emotional lability, comorbidity and impairment in adults with attention-deficit hyperactivity disorder". Journal of Affective Disorders. 147 (1–3): 80–6. doi:10.1016/j.jad.2012.10.011. PMID 23218897.
- Latoo, Javed; Mistry, Minal; Dunne, Francis J (2013). "Often overlooked neuropsychiatric syndromes in Parkinson's disease". British Journal of Medical Practitioners. 6 (1). Archived from the original on 4 November 2014. Retrieved 4 November 2014.
- Levitt, S. (2013). Treatment of Cerebral Palsy and Motor Delay. Wiley. ISBN 9781118699782. Archived from the original on 20 February 2017. Retrieved 4 November 2014.
- Atchison, B.; Dirette, D.K. (2007). Conditions in Occupational Therapy: Effect on Occupational Performance. Lippincott Williams & Wilkins. p. 40. ISBN 9780781754873. Archived from the original on 20 February 2017. Retrieved 4 November 2014.
- Devinsky, O; Vazquez, B (1993). "Behavioral changes associated with epilepsy". Neurological Clinics. 11 (1): 127–49. doi:10.1016/S0733-8619(18)30173-7. PMID 8441366.
- "Medscape: Medscape Access". medscape.com. Archived from the original on 2 November 2015. Retrieved 4 November 2014.
- Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA; Gresham; Bromberg; Kasarkis; Smith (1997). "A self report measure of affective lability". Journal of Neurology, Neurosurgery, and Psychiatry. 63 (1): 89–93. doi:10.1136/jnnp.63.1.89. PMC 2169647. PMID 9221973.CS1 maint: multiple names: authors list (link)
- Shaibani AT, Sabbagh MN, Doody R (1994). "Laughter and crying in neurologic disorders". Neuropsychiatry, Neuropsychology & Behavioral Neurology. 7: 243–250.
- Black DW (1982). "Pathological laughter. A review of the literature". Journal of Nervous and Mental Disease. 170 (2): 67–71. doi:10.1097/00005053-198202000-00001. PMID 7057172.
- Green RL (1998). "Regulation of affect". Seminars in Clinical Neuropsychiatry. 3 (3): 195–200. PMID 10085207.
- Cummings J, Arciniegas D, Brooks B, Herndon R, Lauterbach E, Pioro E, Robinson R, Scharre D, Schiffer R, Weintraub D; Arciniegas; Brooks; Herndon; Lauterbach; Pioro; Robinson; Scharre; Schiffer; Weintraub (2006). "Defining and diagnosing involuntary emotional expression disorder". CNS Spectrums. 11 (6): 1–7. doi:10.1017/S1092852900026614. PMID 16816786.CS1 maint: multiple names: authors list (link)
- Archiniegas DB, Topkoff J (2000). "The neuropsychiatry of pathologic affect: an approach to evaluation and treatment". Seminars in Clinical Neuropsychiatry. 5 (4): 290–306. doi:10.1053/scnp.2000.9554. PMID 11291026.
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR; Anderson; Martin; Damasio; Damasio (2001). "Pathological laughter and crying: a link to the cerebellum". Brain. 124 (Pt 9): 1708–1719. doi:10.1093/brain/124.9.1708. PMID 11522574.CS1 maint: multiple names: authors list (link)
- McCullagh S, Moore M, Gawel M, Feinstein A (1999). "Pathological laughing and crying in amyotrophic lateral sclerosis: an association with prefrontal cognitive dysfunction". Journal of the Neurological Sciences. 169 (1–2): 43–48. doi:10.1016/s0022-510x(99)00214-2. PMID 10540006.
- Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR; Parikh; Lipsey; Starkstein; Price (1993). "Pathological laughing and crying following stroke: validation of a measurement scale and a double-blind treatment study". American Journal of Psychiatry. 150 (2): 286–293. doi:10.1176/ajp.150.2.286. PMID 8422080.CS1 maint: multiple names: authors list (link)
- Lopez, Oscar L.; Gonzalez, Maria P.; Becker, James T.; Reynolds, Charles F.; Sudilovsky, Abraham; DeKosky, Steven T. (1996). "Symptoms of depression and psychosis in Alzheimer's disease and frontotemporal dementia: exploration of underlying mechanisms". Neuropsychiatry, Neuropsychology & Behavioral Neurology. 9 (3): 154–161.
- Tremont Geogffery. "Neurbehavioral Functioning in Thyroid Disorders". Medicine and Health."Archived copy" (PDF). Archived (PDF) from the original on 2015-12-23. Retrieved 2015-06-17.CS1 maint: archived copy as title (link)
- Greenamyre JT (1986). "The role of glutamate in neurotransmission and in neurologic disease". Archives of Neurology. 43 (10): 1058–1063. doi:10.1001/archneur.1986.00520100062016. PMID 2428340.
- Bittigau P, Ikonomidou C; Ikonomidou (1997). "Glutamate in neurologic diseases". Journal of Child Neurology. 12 (8): 461–485. doi:10.1177/088307389701200802. PMID 9430311.
- Mattson MP (2003). "Excitotoxic and excitoprotective mechanism: abundant targets for the prevention and treatment of neurodegenerative disorders". Neuromolecular Medicine. 3 (2): 65–94. doi:10.1385/NMM:3:2:65. PMID 12728191.
- House A, Dennis M, Molyneux A, Warlow C, Hawton K; Dennis; Molyneux; Warlow; Hawton (1989). "Emotionalism after stroke". BMJ. 298 (6679): 991–994. doi:10.1136/bmj.298.6679.991. PMC 1836312. PMID 2499390.CS1 maint: multiple names: authors list (link)
- Seliger GM, Hornstein A, Flax J, Herbert J, Schroeder K; Hornstein; Flax; Herbert; Schroeder (1992). "Fluoxetine improves emotional incontinence". Brain Injury. 6 (3): 267–270. doi:10.3109/02699059209029668. PMID 1581749.CS1 maint: multiple names: authors list (link)
- Feinstein A, Feinstein K, Gray T, O'Connor P; Feinstein; Gray; O'Connor (1997). "Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis". Archives of Neurology. 54 (9): 1116–1121. doi:10.1001/archneur.1997.00550210050012. PMID 9311355.CS1 maint: multiple names: authors list (link)
- Kim JS (2002). "Post-stroke emotional incontinence after small lenticulocapsular stroke: correlation with lesion location". Journal of Neurology. 249 (7): 805–810. doi:10.1007/s00415-002-0714-4. PMID 12140660.
- Harris Y, Gorelick PB, Cohen D, Dollear W, et al. (1994). "Psychiatric symptoms in dementia associated with stroke: a case-control analysis among predominantly African-American patients". Journal of the National Medical Association. 86 (9): 697–702. PMC 2607578. PMID 7966434.
- Ross ED, Stewart RS; Stewart (1987). "Pathological display of affect in patients with depression and right frontal brain damage. An alternative mechanism". Journal of Nervous and Mental Disease. 175 (3): 165–172. doi:10.1097/00005053-198703000-00007. PMID 3819712.
- Haupt (1996). "Emotional lability, intrusiveness, and catastrophic reactions". International Psychogeriatrics. 8 (Suppl 3): 409–414. doi:10.1017/S1041610297003736. PMID 9154598.
- Sloan RL, Brown KW, Pentland B; Brown; Pentland (1992). "Fluoxetine as a treatment for emotional lability after brain injury". Brain Injury. 6 (4): 315–319. doi:10.3109/02699059209034945. PMID 1638265.CS1 maint: multiple names: authors list (link)
- Surridge D (1969). "An investigation into some psychiatric aspects of multiple sclerosis". British Journal of Psychiatry. 115 (524): 749–764. doi:10.1192/bjp.115.524.749. PMID 5806869.
- Caroscio JT, Mulvihill MN, Sterling R, Abrams B; Mulvihill; Sterling; Abrams (1987). "Amyotrophic lateral sclerosis". Neurological Clinics. 5 (1): 1–8. doi:10.1016/S0733-8619(18)30931-9. PMID 3561382.CS1 maint: multiple names: authors list (link)
- Gallagher JP (1989). "Pathologic laughter and crying in ALS: a search for their origin". Acta Neurologica Scandinavica. 80 (2): 114–117. doi:10.1111/j.1600-0404.1989.tb03851.x. PMID 2816272.
- Wicks P Frost J; Frost (2008). "ALS patients request more information about cognitive symptoms". European Journal of Neurology. 15 (5): 497–500. doi:10.1111/j.1468-1331.2008.02107.x. PMID 18325023.
- Zeilig G, Drubach DA, Katz-Zeilig M, Karatinos J; Drubach; Katz-Zeilig; Karatinos (1996). "Pathological laughter and crying in patients with closed traumatic brain injury". Brain Injury. 10 (8): 591–597. doi:10.1080/026990596124160. PMID 8836516.CS1 maint: multiple names: authors list (link)
- "Archived copy". Archived from the original on 2011-09-27. Retrieved 2011-08-16.CS1 maint: archived copy as title (link)
- Fellus JL, Kantor D, Kaye RE (2012). "PRISM registry: A novel research tool to determine the prevalence of pseudobulbar affect". European Journal of Neurology. 19 (S1): 85–90. doi:10.1111/j.1468-1331.2012.03887.x.
- Burns, Alistair; Russell, Eve; Stratton-Powell, Hilary; Tyrell, Pippa; O'Neill, Paul; Baldwin, Robert (1999). "Sertraline in stroke-associated lability of mood". International Journal of Geriatric Psychiatry. 14 (8): 681–5. doi:10.1002/(SICI)1099-1166(199908)14:8<681::AID-GPS49>3.0.CO;2-Z. PMID 10489659.
- Brown, K. W.; Sloan, R. L.; Pentland, B. (1998). "Fluoxetine as a treatment for post-stroke emotionalism". Acta Psychiatrica Scandinavica. 98 (6): 455–8. doi:10.1111/j.1600-0447.1998.tb10119.x. PMID 9879787.
- Andersen, G; Vestergaard, K; Riis, J. O. (1993). "Citalopram for post-stroke pathological crying". The Lancet. 342 (8875): 837–9. doi:10.1016/0140-6736(93)92696-Q. PMID 8104273.
- Robinson, RG; Parikh, RM; Lipsey, JR; Starkstein, SE; Price, TR (1993). "Pathological laughing and crying following stroke: Validation of a measurement scale and a double-blind treatment study". American Journal of Psychiatry. 150 (2): 286–93. doi:10.1176/ajp.150.2.286. PMID 8422080.
- Schiffer, Randolph B.; Herndon, Robert M.; Rudick, Richard A. (1985). "Treatment of Pathologic Laughing and Weeping with Amitriptyline". The New England Journal of Medicine. 312 (23): 1480–2. doi:10.1056/NEJM198506063122303. PMID 3887172.
- "Dextromethorphan (DXM)". Cesar.umd.edu. Archived from the original on 2013-07-26. Retrieved 2016-02-18.
- "Quinidine sulfate". PDRhealth™. 2016. Archived from the original on 2015-09-05. Retrieved 2016-02-18.
- "Label: NUEDEXTA- dextromethorphan hydrobromide and quinidine sulfate capsule, gelatin coated". U.S. National Library of Medicine, DailyMed. 2015-01-30. Archived from the original on 2016-03-03. Retrieved 2016-02-18.
- "Nuedexta, FDA Approval History". Drugs.com. January 2015. Archived from the original on 2016-03-03. Retrieved 2016-02-18.
- "Safety and Efficacy of AVP-923 in PBA Patients With ALS or MS (STAR)". ClinicalTrials.gov. June 2013. Archived from the original on 2016-03-02. Retrieved 2016-02-19.
- Pioro, Erik P.; Brooks, Benjamin Rix; Cummings, Jeffrey; Schiffer, Randolph; Thisted, Ronald A.; Wynn, Daniel; Hepner, Adrian; Kaye, Randall (2010). "Dextromethorphan Plus Ultra Low-Dose Quinidine Reduces Pseudobulbar Affect". Annals of Neurology. 68 (5): 693–702. doi:10.1002/ana.22093. PMID 20839238.
- Archiniegas DB, Lauterbach EC, Anderson KE, Chow TW, et al. (2005). "The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting". CNS Spectrums. 10 (5): 1–16. doi:10.1017/S1092852900026602. PMID 15962457.
- Brooks, Benjamin Rix; Crumpacker, David; Fellus, Jonathan; Kantor, Daniel; Kaye, Randall E (Aug 21, 2013). "PRISM: A Novel Research Tool to Assess the Prevalence of Pseudobulbar Affect Symptoms across Neurological Conditions". PLOS ONE. Public Library of Science. 8 (8): e72232. Bibcode:2013PLoSO...872232B. doi:10.1371/journal.pone.0072232. PMC 3749118. PMID 23991068.
- "Archived copy". Archived from the original on 2013-11-18. Retrieved 2013-10-26.CS1 maint: archived copy as title (link)
- p. 156 "Archived copy". Archived from the original on 2013-11-18. Retrieved 2013-10-26.CS1 maint: archived copy as title (link)
- "Joaquin Phoenix Reveals the Dark, Real World Origin of His Joker's Laugh". CBR. Retrieved 12 October 2019.
External links
- "Pseudobulbar affect and stroke" on the National Stroke Association website
| Classification |
|---|