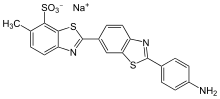
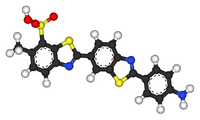
Primuline
Primuline is a dye containing the benzothiazole ring system. Primuline itself is also known as Direct yellow 7, Carnotine, or C.I. 49010.
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.698 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H15N3O3S3 (free acid) | |
| Molar mass | 453.557 g/mol (free acid) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The primulines are considered derivatives of dehydrothiotoluidine (aminobenzenyltoluylmercaptan), which is obtained when para-toluidine is heated with sulfur for eighteen hours at 180–190 °C and then for a further six hours at 200–220 °C[1] Dehydrothiotoluidine is not itself a dye-stuff, but if the heating is carried out at a higher temperature in the presence of more sulfur, then a base is formed, which gives primuline yellow upon sulfonation.[2]
Primuline yellow is a mixture of sodium salts and probably contains at least three thiazole rings in combination. It is a substantive cotton dye of rather fugitive shade, but can be diazotized on the fibre and then developed with other components, yielding a series of ingrain colors.[3]
Primuline is usually available as a sodium salt. Primuline is fluorescent.
Thioflavin T is obtained by the methylation of dehydrothiotoluidine with methanol in the presence of hydrochloric acid. Thioflavin S results from the methylation of dehydrothiotoluidine with sulfonic acid. This sulfonic acid on oxidation with bleaching powder or with lead peroxide, in alkaline solution yields chloramine yellow, which dyes cotton a beautiful yellow.[3]
References
- P. Jacobson (1889). "N/A". Ber. 22: 333.
L. Gatterrnann, ibid. p. 1084 - A. G. Green (1888). "N/A". J. Soc. Chem. Ind. 1: 194.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 22 (11th ed.). Cambridge University Press. p. 342.