Polyvalency (chemistry)
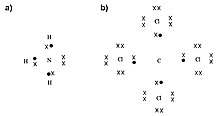
In chemistry, polyvalency (or polyvalence, multivalency) is used to describe chemical species, generally atoms or molecules, which exhibit more than one valence (chemistry) (Fig. 1).[1][2][3] Species that are polyvalent can form multiple chemical bonds, the number of which is dictated by their valency. In other words, bivalent species can form two bonds, trivalent species can form three bonds, and so on.[4] The principle of polyvalency also applies to larger species, such as antibodies, medical drugs, and even nanoparticles surface-functionalized with ligands, like spherical nucleic acids, which can show enhanced or cooperative binding compared to their monovalent counterparts.[5][6][7][8] It has been shown that nanoparticles with multiple nucleic acid strands on their surfaces (e.g., DNA) have the ability to form multiple bonds with one another via DNA hybridization to form hierarchical assemblies, some of which are highly crystalline in nature.[9]
References
- Vance, David; Shah, Mrinal; Joshi, Amit; Kane, Ravi S. (15 October 2008). "Polyvalency: a promising strategy for drug design". Biotechnology and Bioengineering. National Center for Biotechnology Information. pp. 429–434. doi:10.1002/bit.22056. PMID 18727104. Retrieved 2020-08-04.
- Wu, Albert M.; Wu, June H.; Liu, Jia-Hau; Singh, Tanuja; André, Sabine; Kaltner, Herbert; Gabius, Hans-Joachim (April 2004). "Effects of polyvalency of glycotopes and natural modifications of human blood group ABH/Lewis sugars at the Galbeta1-terminated core saccharides on the binding of domain-I of recombinant tandem-repeat-type galectin-4 from rat gastrointestinal tract (G4-N)". Biochimie. National Center for Biotechnology Information. pp. 317–326. doi:10.1016/j.biochi.2004.03.007. PMID 15194236. Retrieved 2020-08-04.
- Kane, Ravi (2006-11-01). "Polyvalency: Recent developments and new opportunities for chemical engineers". AIChE Journal. Researchgate. pp. 3638–3644. doi:10.1002/aic.11011. Retrieved 2020-08-04.
- Cartmell, E.; Fowles, G. W. A. (1983). Valency and Molecular Structure (4th ed.). ISBN: 0-408-70809-3.
- Crothers, D.; Metzger, H. (1972). “The influence of polyvalency on the binding properties of antibodies”. Immunochemistry. 9 (3): 341-357. doi: 10.1016/0019-2791(72)90097-3
- Davis, K. A.; et al. (1999). “Determination of CD4 antigen density on cells: Role of antibody valency, avidity, clones, and conjugation”. Cytometry Part A. 33 (2):197–205. doi: 10.1002/(SICI)1097-0320(19981001)33:2<197::AID-CYTO14>3.0.CO;2-P
- Jones, M. A.; et al. (2015) “Programmable materials and the nature of the DNA bond”. Science. 347 (6224): 840. doi: 10.1126/science.1260901
- Hu, X.; et al. (2019) “Valency-Controlled Molecular Spherical Nucleic Acids with Tunable Biosensing Performances”. Anal. Chem. 91 (17): 11374–11379. doi: 10.1021/acs.analchem.9b02614
- Macfarlane, R. J.; et al. (2011). "Nanoparticle Superlattice Engineering with DNA". Science. 334 (6053): 204–208. doi:10.1126/science.1210493.